 With growing awareness of the many roles played by vitamin D metabolites, testing volume is increasing
With growing awareness of the many roles played by vitamin D metabolites, testing volume is increasing
BY CRAIG FOREBACK, PHD
Vitamin D and its various forms are better characterized as hormones or prohormones rather than as a vitamin since the active form has many regulatory functions. An abundance of published clinical trials and epidemiological studies have contributed to the growing awareness of the many roles played by vitamin D metabolites. Vitamin D status is linked not only to classical targets such as bone, kidney, and intestine, but to an extensive diversity of tissues.1
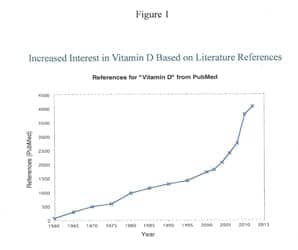
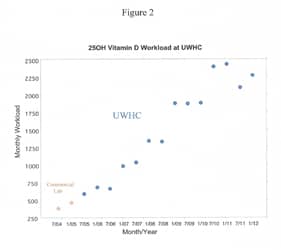
Health care practitioners are now seeing the benefits of assessing vitamin D levels among patients of all ages and health statuses on a frequent basis. It is no surprise that there has been a surge of vitamin D testing in recent years (Figure 1). Likewise, the number of publications related to vitamin D testing has increased almost exponentially over the last 10 years (Figure 2).
This has led to the realization that an alarming number of people are vitamin D-deficient or have insufficient levels of vitamin D. Insufficient levels of vitamin D has been related to reduced immunological response, various forms of cancer, heart diseases, diabetes, rheumatoid arthritis, cognitive impairment, and overall mortality.2 One recent article reported an inverse relationship between epidemic influenza and solar radiation.3 The most recent newsletter from the Vitamin D Council (May 7, 2013)4 reviewed the research on multiple sclerosis (MS) and vitamin D. The link between when and where you were born and MS is well established. MS is more common in areas well north of the equator and in people born in early spring, suggesting that the amount of sunshine you get may be a risk factor for MS. Might this link be vitamin D-related?
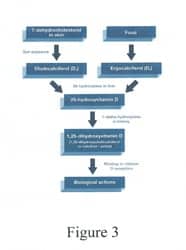
Vitamin D is a steroid-derived, fat-soluble vitamin. The vitamin and its metabolites exist in two forms: D2 and D3. Those identified as D2 are derived from yeast and plant material that has been irradiated with UVB light. It is available through supplementation and fortification of foods (eg, milk). D3 is found in foods and supplements, and it can be produced by the body. The vitamin and its metabolites are transported in the blood bound to vitamin D-binding protein (DBP). Although similar in structure, D2 derivatives have been reported to be 30% to 50% as active as D3 forms in the maintenance of serum 25-hydroxy vitamin D levels. However, some sources refute this claim.
Vitamin D is one of a few vitamins that can be produced by the body. Cutaneous 7-dehydrocholesterol undergoes photolysis to cholecalciferol when skin is exposed to UVB radiation. Twenty minutes of summer sunlight exposure can produce 20,000 units of Vitamin D3.5 The active form of vitamin D is produced by two sequential hydroxylations in the liver, then the kidneys (Figure 3). However, the latitude has a dramatic effect on this conversion. During the winter months at or above 35° N latitude, there is insufficient UVB to produce sufficient cholcalciferol to maintain adequate 25-hydroxy vitamin D. The vast majority (two-thirds) of residents in the continental United States and Alaska live at or above the above the 35th parallel. Because of the diminished sunlight from fall through early spring in most locales, increased intake of dietary vitamin D is critical. Fatty fish provides an excellent source. Fortified foods provide most of the vitamin D in the American diet, as there is modest consumption of foods naturally high in vitamin D. When UVB exposure is insufficient, supplementation is recommended.
TESTING FOR VITAMIN D
The first practical assay for vitamin D was a radioimmunoassay developed by DiaSorin, Saluggia, Italy, in 1985.6 The method had a minimum of simple, sample-handling steps, and was easy to automate. It compared favorably with high-performance liquid chromatography (HPLC) for the measurement of total vitamin D, but it was not able to discriminate between D2 and D3.
HPLC/UV
An HPLC method with UV detection that was less technically demanding and had lower costs than HPLC/MS/MS was developed at the University of Wisconsin Hospital and Clinics (UWHC) Laboratory, Madison, Wis, in 2005, and was reported in Clinical Chemistry by Lensmeyer et al.7 It is still in use at UWHC, although researchers are evaluating HPLC with tandem MS for the determination of 1,25-hydroxyvitamin D as well as several other vitamin D3 and D2 metabolites. The method can differentiate vitamin D2, D3, and C-3 epimer of 25-hydroxyvitamin D3, and it is effective for clinical evaluation of vitamin D levels in clinical settings. It has also been effectively transferred to two other laboratories.
IMMUNOASSAYS
Immunoassays are extensively used and have been the basis of large studies such as the National Health and Nutrition Examination Survey, the Harvard Nurses’ study, the Harvard Health Professions Study, and the Framingham Study. One large reference lab (LabCorp, Burlington, NC) employs an immunoassay for its routine 25-hydroxyvitamin D assay. Modern immunoassays use chemiluminescent labels for detection.
At least five different manufacturers have automated platforms with immunoassays for the testing of 25-hydroxy vitamin D, including Abbott, Abbott Park, Ill (ARCHITECT); DiaSorin Inc, with US headquarters in Stillwater, Minn (LIAISON); Roche, Indianapolis (Elecsys Systems); Siemens Healthcare (ADVIA Centaur); and Immunodiagnostic Systems Limited, UK (IDS-iSYS). Recent studies seeking to compare assays reported differences in the 25-OH vitamin D values observed across platforms and manufacturers.8,9 Today’s assays are required to measure both the vitamin D2 and D3 forms to accurately reflect total blood levels. This is particularly important for patients who are taking prescription supplements (which in some countries are in the form of vitamin D2) because a partial 25-OH vitamin D measurement could erroneously under-report therapeutic or even toxic circulating vitamin D levels.
Not all immunoassays recognize vitamin D2. It is important to choose an assay that has equal detection of vitamin D2 and D3 for routine clinical lab use. A recent report suggested that serum concentrations of vitamin D binding protein could cause bias of the reported vitamin D value by certain immunoassays.8 This report also found that certain immunoassays for some as yet unexplained reason under-report vitamin D levels in hemodialysis patients.
ID-XLC-MS/MS
Isotope dilution-extraction tandem mass spectroscopy has created the opportunity for routine clinical laboratories to return to a rigorous method in which sample extraction, chromatography, and correction for procedural losses have been restored and specificity enhanced. LC/MS/MS is now widely used as the reference method in comparison studies and evaluation of a new method for vitamin D assays.
THE SAGA OF THE C-3 EPIMER OF 25-HYDROXYVITAMIN D3
As stated in the beginning of this article, measurement of circulating 25-hydroxyvitamin D has been subject to substantial laboratory and methodological variation. Recent assay improvements and the development of standards have enhanced agreement among laboratories and methods. Despite these advances, it remains that unappreciated factors continue to confound 25-hydroxyvitamin D measurements. The reported presence of the C-3 epimer of 25-hydroxyvitamin D3 in human serum10,11 is one such confounder. Some HPLC-MS/MS do not separate this metabolite, most likely due to inadequate run times.
It is widely believed that the C-3 epimer is only found in neonates, so the lack of separation of the two molecules with many LC/UV methods or LC/MS/MS methods was thought not to be a problem. Lensmeyer et al12 developed highly sensitive methods for the measurement of 3-epi 25 hydroxyvitamin D3 and established the presence of this isomer in adult serum. A recent report from Phenomenex Inc, Torrance, Calif,13 describes a method that provides excellent resolution of all on the monohydroxy D3 isomers, including the 3-epi within a short run time. In many patients the levels of this isomer are significant and can lead to an inappropriate determination of a patient’s vitamin D status.
THE FUTURE
The laboratory at UWHC is currently using LC/MS/MS to develop a method for the simultaneous separation and quantitation of a wide variety of vitamin D metabolites, including the C-3 epimer, 24,25-hydroxyvitamin D3 and D2, as well as the sulfated forms of the vitamin. According to Don Wiebe, PhD, technical director of clinical chemistry and toxicology, UWHC, the determination of total vitamin D levels alone will not be adequate for the assessment of a patient’s vitamin D status. He proposes that a panel of vitamin D metabolites may be necessary for at least the initial assessment of each patient and even for follow-up studies.
Craig Foreback, PhD, is a contributing writer for CLP. For more information, contact Editor Judy O’Rourke, [email protected].
REFERENCES
1. Norman AW. From vitamin D to hormone D; Fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2006;88;491S-499S.
2. Wiebe DA. Vitamin D methods: what’s all the fuss? 2013 ASCLS-WI Annual Meeting; April 24-25; Madison Wis.
3. Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006;134(6):1129-1140.
4.Vitamin D Council Newsletter, May 7, 2013. Available at: http//:www.vitamindcouncil.org. Accessed May 17, 2013.
5. Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Alt Med Rev. 2008;13:6-20.
6. Hollis BW, Napoli JL. Determination of vitamin D status by radioimmunoassay with an I 125 labeled tracer. Clin Chem. 1985;31(11):529-533.
7. Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: Comparison with contemporary assays. Clin Chem. 2006;52(6):1120-1126.
8. Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clin Chem. 2012;58:543-48.
9. Farrell CJL, Martin S, Mcwinnnney B, et al. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography–tandem mass spectrometry methods. Clin Chem. 2012;58:531-543.
10. Schleicher RL, Encisco SE, Madhulika C-W, et al. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chem Acta. 2011;412:1594-1599.
11. Strathmann FG, Sadilkova K, Laha TJ, et al. 3-epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chem Acta. 2012;413:203-206.
12. Lensmeyer G, Poquette M, Wiebe D, Binkley N. The C-3 Epimer of 25-hydroxyvitamin D3 is present in adult serum. J Clin Endocrinol Metab. 2012;97(1):163-168.
13. Sadjedi S, Layne J. Phenomenex Applications, Phenomenex Inc, Torrance, CA 90501, www.phenomenex.com.
CLICK HERE for a pdf of of a related journal article, co-authored by Marcel J.W. Janssen.
|
VITAMIN D TESTING PRODUCTS
|
|
|


 AUDIT MicroControls Inc, Carlsbad, Calif, has received 510(k) approval of Vitamin D as an addition to its complete line of calibration verification/linearity and daily quality control products. The Serum based Vitamin D Linearity & Control Kits will become available fall 2013. The Vitamin D Linearity will have 5 levels, the control will have 2 levels. This liquid product, when stored at 2° to 8°C has a shelf life of 2 years and an open-vial stability of 14 days for linearity and 30 days for the control. The company provides a free, online, real-time data reduction program that offers Levy-Jennings charts and peer group analysis for daily quality control product users, as well as linearity graphs and peer group analysis. AUDIT MicroControls Inc
AUDIT MicroControls Inc, Carlsbad, Calif, has received 510(k) approval of Vitamin D as an addition to its complete line of calibration verification/linearity and daily quality control products. The Serum based Vitamin D Linearity & Control Kits will become available fall 2013. The Vitamin D Linearity will have 5 levels, the control will have 2 levels. This liquid product, when stored at 2° to 8°C has a shelf life of 2 years and an open-vial stability of 14 days for linearity and 30 days for the control. The company provides a free, online, real-time data reduction program that offers Levy-Jennings charts and peer group analysis for daily quality control product users, as well as linearity graphs and peer group analysis. AUDIT MicroControls Inc UTAK, Valencia, Calif, uses a 100% real human serum matrix to manufacture its vitamin D controls, which enables them to be utilized with all contemporary reference methods and immunoassays. The company does not add any preservatives, it does not use stripped serum or human-based serum, and it does not fortify its serum matrix pools with horse serum. The company’s quality control material more closely mimics the human patient specimen and virtually eliminates matrix effects.
UTAK, Valencia, Calif, uses a 100% real human serum matrix to manufacture its vitamin D controls, which enables them to be utilized with all contemporary reference methods and immunoassays. The company does not add any preservatives, it does not use stripped serum or human-based serum, and it does not fortify its serum matrix pools with horse serum. The company’s quality control material more closely mimics the human patient specimen and virtually eliminates matrix effects. for use on Tosoh AIA System Analyzers.* Vitamin D measurement is intended as an aid in the determination of Vitamin D sufficiency. Using the company’s unit dose test cup reagent technology, this product has an assay time of approximately 40 minutes. Single, unitized test cups require no premixing, no premeasuring, and no onboard refrigeration. Dry reagent format ensures 90-day calibration stability for minimal waste and cost-effective testing. Test cups are bar-coded for easy identification and inventory management. This assay is designed for the quantitative measurement of total 25-hydroxyvitamin D (25-OH Vitamin D) in human serum, Na heparinized or EDTA plasma on Tosoh AIA System Analyzers. * ST AIA-PACK 25-OH Vitamin D is not available for use on the AIA-360.
for use on Tosoh AIA System Analyzers.* Vitamin D measurement is intended as an aid in the determination of Vitamin D sufficiency. Using the company’s unit dose test cup reagent technology, this product has an assay time of approximately 40 minutes. Single, unitized test cups require no premixing, no premeasuring, and no onboard refrigeration. Dry reagent format ensures 90-day calibration stability for minimal waste and cost-effective testing. Test cups are bar-coded for easy identification and inventory management. This assay is designed for the quantitative measurement of total 25-hydroxyvitamin D (25-OH Vitamin D) in human serum, Na heparinized or EDTA plasma on Tosoh AIA System Analyzers. * ST AIA-PACK 25-OH Vitamin D is not available for use on the AIA-360. requirement for good quality control has never been more important. The Acusera Immunoassay Speciality I control from Randox Laboratories-US Ltd, Kearneysville, WVa, is designed to accurately monitor the analytical performance of a wide variety of Vitamin D assays. As a true third-party control assay, method-specific target values and ranges are provided for 10 parameters in total, including 1-25-(OH)2-Vitamin D and 25-OH-Vitamin D. The control material is manufactured from 100% human serum, not only providing a matrix similar to the patient sample but minimizing antibody cross-reactivity. A reconstituted stability of up to 5 days at +2°C to 8°C and 4 weeks at -20°C helps to reduce waste and keep costs low.
requirement for good quality control has never been more important. The Acusera Immunoassay Speciality I control from Randox Laboratories-US Ltd, Kearneysville, WVa, is designed to accurately monitor the analytical performance of a wide variety of Vitamin D assays. As a true third-party control assay, method-specific target values and ranges are provided for 10 parameters in total, including 1-25-(OH)2-Vitamin D and 25-OH-Vitamin D. The control material is manufactured from 100% human serum, not only providing a matrix similar to the patient sample but minimizing antibody cross-reactivity. A reconstituted stability of up to 5 days at +2°C to 8°C and 4 weeks at -20°C helps to reduce waste and keep costs low. Poway, Calif, measures total true 25-Hydroxy Vitamin D levels (D2 + D3 levels) in both serum and plasma samples. The assay fully automates the removal of nonspecific protein binding and cross reactivity that may occur in some assays, thereby assuring results will be precise and accurate. The assay is fast and flexible, with complete testing results in under 2 hours. The test is user-friendly and can be used manually or easily adapted for use on a wide range of fully automated microtiter plate readers, making it suitable for use in laboratories of all sizes and testing needs.
Poway, Calif, measures total true 25-Hydroxy Vitamin D levels (D2 + D3 levels) in both serum and plasma samples. The assay fully automates the removal of nonspecific protein binding and cross reactivity that may occur in some assays, thereby assuring results will be precise and accurate. The assay is fast and flexible, with complete testing results in under 2 hours. The test is user-friendly and can be used manually or easily adapted for use on a wide range of fully automated microtiter plate readers, making it suitable for use in laboratories of all sizes and testing needs. pretreatment step that allows for easy automation on virtually any open ELISA processor. The competitive assay strongly correlates with established LC/MS, EIA, and chemiluminescence methods, and offers high sensitivity (1.5 ng/mL) and specificity (D3 – 100%, D2 – 83%) over a wide dynamic range (0-180 ng/mL). Because of the simple pretreatment step and automation capability, the company’s 25-OH Vitamin D provides fast and consistent results. All assay protocol steps are completed in the standard 96-well microtiter plate, without the need for labor-intensive, time-consuming, and complicated pretreatment done manually outside of the analyzer. FDA clearance for the 25-OH Vitamin D, Total ELISA is expected in June 2013.
pretreatment step that allows for easy automation on virtually any open ELISA processor. The competitive assay strongly correlates with established LC/MS, EIA, and chemiluminescence methods, and offers high sensitivity (1.5 ng/mL) and specificity (D3 – 100%, D2 – 83%) over a wide dynamic range (0-180 ng/mL). Because of the simple pretreatment step and automation capability, the company’s 25-OH Vitamin D provides fast and consistent results. All assay protocol steps are completed in the standard 96-well microtiter plate, without the need for labor-intensive, time-consuming, and complicated pretreatment done manually outside of the analyzer. FDA clearance for the 25-OH Vitamin D, Total ELISA is expected in June 2013. enables labs to consolidate vitamin D testing with routine testing a on a single, fully automated immunoassay platform and is traceable to LC/MS/MS (liquid chromatography-mass spectrometry). The assay measures a patient’s total 25(OH) vitamin D level – ~100% of 25 (OH) vitamin D2 and D3, metabolites of the two major forms of vitamin D, in serum or plasma. Measurement of [25(OH)D] is the most widely used indicator of vitamin D status. The assay provides results in less than 18 minutes with a throughput of 240 tests per hour without impacting other routine, stat, or specialty assays.
enables labs to consolidate vitamin D testing with routine testing a on a single, fully automated immunoassay platform and is traceable to LC/MS/MS (liquid chromatography-mass spectrometry). The assay measures a patient’s total 25(OH) vitamin D level – ~100% of 25 (OH) vitamin D2 and D3, metabolites of the two major forms of vitamin D, in serum or plasma. Measurement of [25(OH)D] is the most widely used indicator of vitamin D status. The assay provides results in less than 18 minutes with a throughput of 240 tests per hour without impacting other routine, stat, or specialty assays. automated, chemiluminescent assay that measures both 25 OH Vitamin D2 and D3 for a total vitamin D result. The assay has less than 2% cross-reactivity with the 25 OH Vitamin D C-3 epimer and proven sensitivity down to 4 ng/mL. With a LIAISON XL throughput of 170 tests per hour, the assay can meet the needs of high-volume laboratories, while the benchtop LIAISON analyzer can meet the needs of the medium and small laboratories with an open-kit stability of 4 weeks (at 2? to 8?, dark).
automated, chemiluminescent assay that measures both 25 OH Vitamin D2 and D3 for a total vitamin D result. The assay has less than 2% cross-reactivity with the 25 OH Vitamin D C-3 epimer and proven sensitivity down to 4 ng/mL. With a LIAISON XL throughput of 170 tests per hour, the assay can meet the needs of high-volume laboratories, while the benchtop LIAISON analyzer can meet the needs of the medium and small laboratories with an open-kit stability of 4 weeks (at 2? to 8?, dark). Roche Diagnostics, Indianapolis, offers a fully automated vitamin D test for use on cobas® modular platforms, further expanding the company’s bone metabolism test menu. The Elecsys® Vitamin D assay measures both vitamin D2 and D3, which is important for physicians who have patients taking different forms of vitamin D supplements. Test results are obtained using Roche’s patented electrochemiluminescence (ECL) detection technology, which provides a broad measuring range and high precision at the low end of detection to aid in the assessment of severely deficient patients.
Roche Diagnostics, Indianapolis, offers a fully automated vitamin D test for use on cobas® modular platforms, further expanding the company’s bone metabolism test menu. The Elecsys® Vitamin D assay measures both vitamin D2 and D3, which is important for physicians who have patients taking different forms of vitamin D supplements. Test results are obtained using Roche’s patented electrochemiluminescence (ECL) detection technology, which provides a broad measuring range and high precision at the low end of detection to aid in the assessment of severely deficient patients.