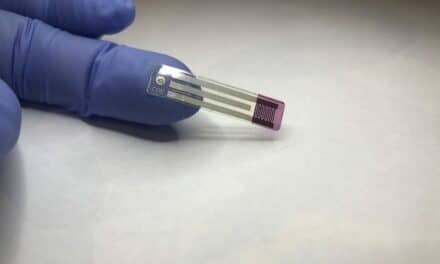
Selux Diagnostics, a biotech startup combating superbug infections and antimicrobial resistance (AMR), received 510(k) clearance from the U.S. Food and Drug Administration for its proprietary Next Generation Phenotyping (NGP) System, a rapid antibiotic susceptibility (AST) testing platform that provides targeted therapeutic results days faster than the current standard of care.
The FDA clearance is for Selux Diagnostics’ in vitro antimicrobial resistance test, which determines a bacteria’s susceptibility to 14 specific antimicrobial agents on the Selux Gram-Positive Panel. Selux’s NGP Gram-Negative panel is currently under review by the FDA.
“This clearance represents a significant advance in infectious disease care and the fight to address antibiotic resistance. Our groundbreaking Selux NGP System holds the potential to save lives and decrease overreliance on broad-spectrum antibiotics, a key factor contributing to the rise of superbugs. We thank all our employees, partners, and advisors who contributed to this tremendous accomplishment,” says Steve Lufkin, CEO of Selux Diagnostics.
Experts predict that without a significant change in today’s treatment options, deaths from superbugs will surpass deaths from cancer by 2050, the company says.
“We have been waiting for true innovation in AST technologies to deliver rapid results that are accurate according to the latest FDA guidelines and include newly approved antibiotics,” says James S. Lewis II, PharmD, FIDSA, co-director of antibiotic stewardship at Oregon Health and Science University, and chair, CLSI Antimicrobial Susceptibility Testing Subcommittee. “I am enthusiastic that the Selux NGP System addresses these critical needs and will become an essential tool for directing personalized therapies for infected patients.”
Selux is confronting this global health crisis head-on via antibiotic susceptibility testing, the critical diagnostic test that informs personalized antibiotic therapy.