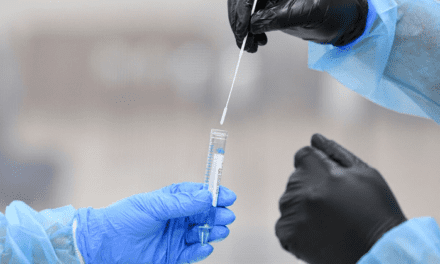
Chembio Diagnostics, Medford, NY, has launched in the United States a rapid DPP Covid-19 serological point-of-care test for the detection of IgM and IgG antibodies. Test results can be obtained within 15 minutes from a simple fingerstick, utilizing Chembio’s MicroReader 1 and MicroReader 2 analyzers.
The ability of the DPP platform to provide numerical results can aid clinicians in determining current or past exposure to the covid-19 virus and monitoring infection progression, while avoiding the human interpretation errors associated with visual readings.
“The results and data from our DPP Covid-19 test can help improve clinical outcomes through the management of individual patients by enabling clinicians to understand the likelihood of past and present infection and to manage populations as a whole as a surveillance test,” says Richard Eberly, chief executive officer of Chembio.
“Our measured approach has positioned us to offer a viable and sustainable long-term solution for clinicians,” adds Eberly. “We expect to begin shipping product in April 2020, and we will continue to work with our partner LumiraDx to provide DPP Covid-19 tests with the ability to scale based upon market demand.”
For more information, visit Chembio Diagnostics.