Engineered recombinant antibodies offer advantages over serum-based controls
By Michael Fiebig, PhD
Serological immunoassays detect specific antibodies in patient samples, with the most relevant example currently being the detection of coronavirus antibodies in covid-19 diagnostic tests. To function correctly, immunoassays rely on calibrators to determine sensitivity and positive controls to ensure an individual test ran correctly. The calibrators and control sera used in diagnostic tests are typically patient-derived, resulting in challenges such as sourcing difficulties, lot-to-lot variability, and undefined characterization.
Coronavirus controls can be particularly hard to obtain from patient material due to biohazard concerns and limited supply. Engineered recombinant antibodies provide a reliable alternative to serum-based controls, offering high batch-to-batch reproducibility for the entire lifespan of an assay, as well as the ability to tailor a control’s isotype or subtype to meet specific assay requirements. Diagnostic developers are therefore choosing to utilize engineered recombinant antibodies in their covid-19 assays. This article will explore the different engineered antibodies being used in covid-19 diagnostic tests and look specifically at new recombinant nanobodies against the SARS-CoV-2 receptor binding domain.
Recombinant Antibody Technology for Diagnostics
Recombinant antibodies are absolutely defined by their amino acid sequence and manufactured in vitro using synthetic genes. Though recombinant antibodies are standard in the pharmaceutical industry, these antibodies have not been as widely available for research and diagnostics applications. This is starting to change, however, due to the benefits recombinant antibodies offer over polyclonal and hybridoma-produced monoclonal antibodies.
Because they are produced in vitro, recombinant antibodies are precisely defined with no variation in binding specificity or affinity. Furthermore, they are not susceptible to supply chain problems such as contamination, genetic drift, or other loss; with recombinant production, the same fully characterized antibody can always be manufactured in unlimited quantities. Recombinant antibodies therefore provide the highest possible batch-to-batch reproducibility for the entire life span of a diagnostic.
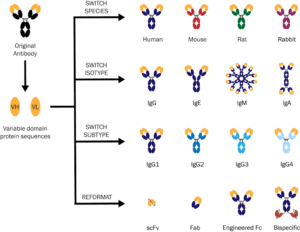
In addition, recombinant production allows for antibody engineering, through which any antibody can be converted into any species, isotype, or subtype. This enables diagnostic developers to tailor their antibody controls to assay requirements, removing the need for a suboptimal pairing that could affect assay sensitivity and background. For example, antibody engineering can be used to standardize all antibody constant domains (also called Fc domains) within an assay to streamline conjugation and immobilization protocols.
Recombinant technologies have enabled the large-scale production of engineered antibodies for diagnostic tests. With antibody sequencing, the exact amino acid sequence of an existing hybridoma cell line or purified monoclonal antibody can be determined (and recently, strides have been made for sequencing polyclonal antibodies as well). Once the amino acid sequence is obtained, the original antibody can be engineered into any species, isotype, or subtype and recombinantly produced with precisely defined protein concentration and product formulation. In the Absolute Antibody laboratory, we use serum-free mammalian transient expression to produce recombinant antibodies in any format at milligram-to-gram scale. Figure 1 illustrates the variety of engineered antibody formats that can be produced for use as serological controls. The different recombinant formats are available off the shelf in our reagents catalog, while our custom antibody services enable diagnostic developers to rapidly reformat and recombinantly express their own antibodies.
Recombinant Controls for Coronavirus
Engineered antibody controls have shown particular utility for the development of covid-19 diagnostic tests. The first wave of diagnostic assays relied on human IgM and human IgG1 coronavirus antibodies, as they typically lead the primary immune response; however, there is more to serology than just those antibody formats. For example, IgA has been shown to dominate the early neutralizing antibody response to SARS-CoV-2,1 while IgG3 appears to be a highly robust marker for a covid-19 immune response.2 With recombinant antibody engineering, any isotype or subtype is easily obtainable. This allows assay developers to select controls tailored to specific covid-19 requirements, rather than building assays around suboptimal antibody formats.
For example, diagnostic developers are blending human SARS-CoV-2 antibodies of different subtypes into precisely defined standards for use as calibrators and controls. First, the different subtypes can be mixed at ratios representative of an average coronavirus patient. This type of standard can be spiked into normal serum to reliably re-create covid-19 patient serum, or it can be used for controlling the performance of serological assays at representative antibody isotype and subtype abundances. The different subtypes can also be combined in equal amounts, which enables the precise calibration of assays seeking to quantify the relative and absolute abundances of the main antibody types induced in covid-19. These types of recombinant SARS-CoV-2 standards aid in the development of precisely calibrated covid-19 assays that remain accurate across a wide range of antibody concentrations. Moreover, this permits setting absolute standards and cutoffs across testing platforms, allowing data from different sources to be integrated into a wider testing and public health strategy.
When the coronavirus pandemic started earlier this year, our laboratory began by repurposing anti-SARS-CoV-1 antibodies for covid-19 research and diagnostics. In early 2020, researchers demonstrated that the anti-SARS spike glycoprotein antibody clone CR3022, first described in 2006, had high affinity for the new coronavirus. Scientists worldwide began exploring it for use as a reagent in covid-19 diagnostic assays, as well as a candidate for mono- or combination-therapy development.
Our team used transient antibody production to quickly manufacture recombinant CR3022 antibodies in all isotypes and subtypes to act as controls in serological tests. Transient expression—a faster, more affordable alternative to stable CHO cell line generation—was vital to rapidly scaling up and meeting global demand for the antibodies. The engineered recombinant CR3022 antibodies have since been shown in peer-reviewed literature to act as positive controls for SARS-CoV-2 antibodies and are now being used in diagnostic kits that test for covid-19 positive patients.
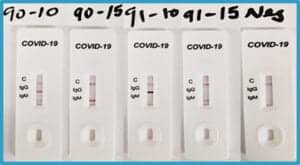
In addition to spike glycoprotein antibodies, anti-SARS nucleoprotein (N) antibodies have also been shown to detect the new coronavirus, in particular the clones CR3009 and CR3018. Again, our laboratory generated engineered versions of the original antibody clones, and they have been utilized as positive controls in covid-19 rapid diagnostic tests (Figure 2). Anti-SARS-CoV-1 antibodies shown to also bind the new SARS-CoV-2 have been extremely valuable for efforts to develop covid-19 diagnostics as quickly as possible. By repurposing existing antibodies, covid-19 research, diagnostics, and therapeutic development were able to move forward while binders specific to SARS-CoV-2 were being generated.
New SARS-CoV-2 Nanobodies
Working in partnership with the University of Zurich, we recently began offering engineered synthetic nanobodies—known as sybodies—against the SARS-CoV-2 receptor binding domain (RBD). Nanobodies are small antibody fragments that can reach previously inaccessible parts of the body due to their compact size. Their better tissue penetration makes nanobodies particularly useful for super-resolution in vivo imaging in research applications, and therapeutic developers are also exploring how to exploit nanobodies’ small size to deliver drugs throughout the human body. For covid-19 therapeutic development, researchers are exploring nanobodies’ potential as inhalable drugs, which would be easier to administer and reach patients’ lungs faster than other treatment formulations.
To generate synthetic nanobodies against the RBD of SARS-CoV-2, the laboratory of Markus Seeger at University of Zurich developed a rapid in vitro selection platform. Within a 2-week timeframe, the lab had identified more than 60 unique anti-RBD sybodies from combinatorial display libraries. Further research showed that six of the sybodies bound SARS-CoV-2 spike protein with very high affinity, while five of those also inhibited ACE2, the host cell receptor to which SARS-CoV-2 binds to initiate the covid-19 infection. Moreover, two of the sybodies can simultaneously bind the RBD, which could enable the construction of a polyvalent antiviral drug.
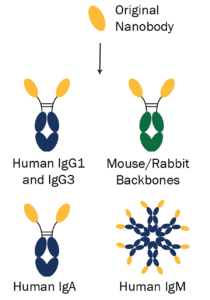
To extend the reach and potential applications of the synthetic nanobodies, the Seeger laboratory partnered with Absolute Antibody. We used antibody engineering to fuse the nanobodies to Fc domains in different species, isotypes, and subtypes (Figure 3). For example, the anti-RBD binders are now available with human IgG1, IgG3, IgM, and IgA domains for use as serological controls in diagnostic assays.
The added Fc domains further extend the applications of the sybodies by varying effector function and permitting increased half-life in in vivo studies. The original nanobodies and newly engineered formats are all recombinantly produced, for ensured batch-to-batch reproducibility, high purity, and low endotoxin levels. The engineered nanobodies with added Fc domains are particularly useful for diagnostic development, as the different isotypes and subtypes provide control choice during assay design.
In addition, recent data from our laboratory has shown that two of the nanobody clones form a matched antibody pair, which is useful for sandwich assays. Sandwich assays use two monoclonal antibodies to improve the specificity of antigen detection assays, by requiring detection of a target via two distinct epitopes. The bridged conformation of the final immune complexes also underpins the design of lateral flow immunoassays or rapid tests. It can often be challenging to find matched antibody pairs from a single source, resulting in complicated and sometimes unstable supply chains. Using two of the SARS-CoV-2 nanobodies prevents that problem and enables improved performance in antigen detection assays.
Recombinant Technology for All
Absolute Antibody was founded with a vision to make engineered recombinant antibodies available to all, in particular for research and diagnostic applications where access to recombinant antibody technology is typically rare. For diagnostic developers, recombinant antibodies provide batch-to-batch reproducibility for the entire life span of an assay, as well as the ability to develop complex controls with absolute precision.
When the covid-19 pandemic began, we used our antibody engineering and recombinant production technology to quickly provide antibody controls for diagnostic developers around the world. We harnessed the potential of existing antibodies, such as the CR3022 clone, as well as partnered with academic laboratories to offer new SARS-CoV-2 binders in engineered formats.

New developments keep occurring; for example, we recently began offering SARS-CoV-2 neutralizing antibodies derived from the blood cells of a covid-19 infected patient. The antibodies were originally generated by Fred Hutchinson Cancer Research Center, and we engineered them into new species, isotypes, and subtypes. We hope that unique antibody controls such as these and the engineered nanobodies provide researchers and diagnostic developers with the tools they need to continue the global fight against the coronavirus pandemic. l
Michael Fiebig, PhD, is vice president of product portfolio and innovation at Absolute Antibody. Fiebig studied biochemistry before obtaining his doctoral degree at the Sir William Dunn School of Pathology, University of Oxford. He joined Absolute Antibody in 2014 and is responsible for the catalog and new applications of Absolute Antibody’s technologies across the research reagents and diagnostics markets.
References
1. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. medRxiv 2020.06.10. doi: 10.1101/2020.06.10.20126532.
2. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033-1036. doi:10.1038/s41591-020-0913-5.
Featured image: A laboratory within the Absolute Antibody manufacturing facility.