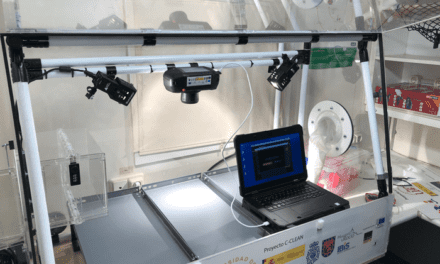
The CDC has issued Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19), which it has recently updated.
Revisions were made on November 3, 2020 to reflect clarification on language for the collection of anterior nasal specimens. Revisions were made on October 8, 2020 to reflect clarification on language for the collection of specimens and to include the addition of saliva language.
For more extensive details, read the entire document at the CDC.