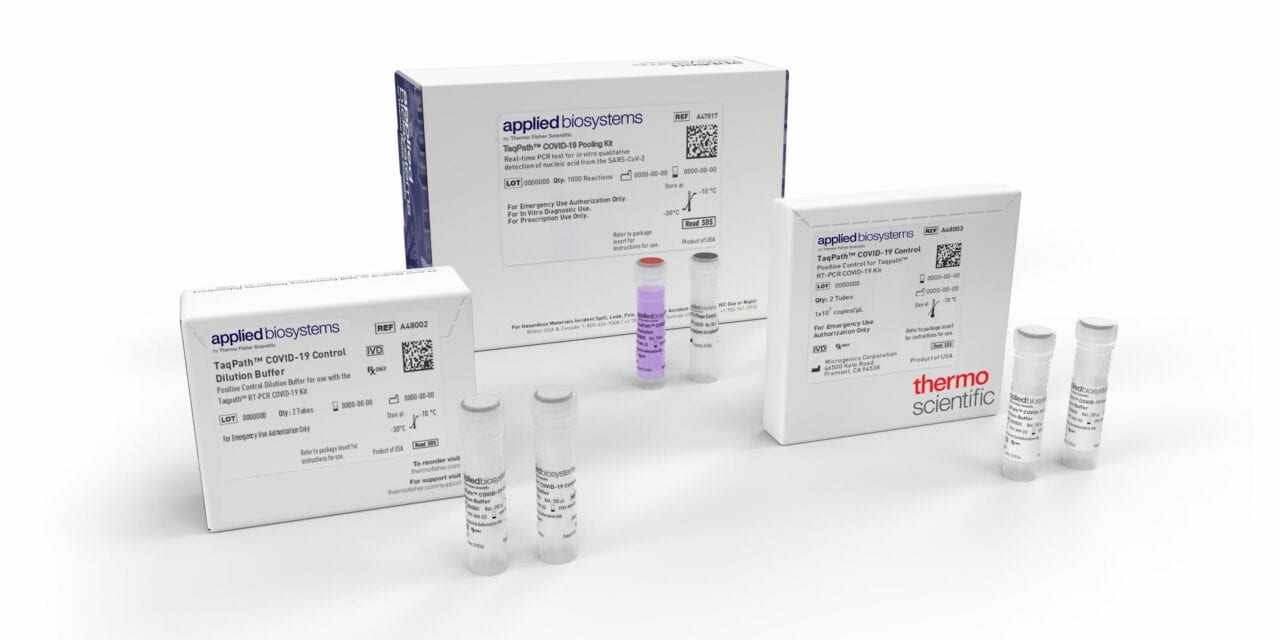
Thermo Fisher Scientific, Waltham, Mass., announced that the FDA has granted emergency use authorization (EUA) for the Applied Biosystems TaqPath COVID-19 Pooling Kit, a highly sensitive real-time PCR multiplex test that enables laboratories to increase testing efficiencies and decrease time to deliver results.
The test allows labs to detect the presence of SARS-CoV-2 in up to five samples simultaneously using a single detection kit for diagnostic population testing in areas with low incidence rates.
The solution is highly sensitive with a level of detection down to 50 copies/mL and includes interpretive software that converts genetic analysis data into a readable report to reduce risk of interpretation error. The kit contains the assays and controls for the qualitative detection of nucleic acid from SARS-CoV-2 in pooled upper respiratory specimens such as nasopharyngeal, oropharyngeal, nasal, and mid-turbinate swabs, and nasopharyngeal aspirate from individuals suspected of COVID-19.
Features and benefits of the TaqPath COVID-19 Pooling Kit include:
- Five samples in a single reaction
- Multiuse in both pool testing and the deconvolution of positive pools
- Multitarget assay design that compensates for emerging SARS-CoV-2 variants and mutations
- Compatible with existing menu and infrastructure
- Part of a complete clinical solution with Applied Biosystems COVID-19 Interpretive Software, which converts genetic analysis data into a readable report
For more information, visit Thermo Fisher Scientific.