Through much of 2020, SARS-CoV-2 accumulated mutations at a slow and steady rate. However, as global cases approached 100 million by the end of the year, more extensive variants began to emerge, exhibiting new pathogenic traits.
By Andy Lane, PhD
As SARS-CoV-2 began its global proliferation in early 2020, scientists hastened to investigate its biology, develop diagnostic tests, and design candidate vaccines, marking one of the most research-intensive periods in human history. Aside from where the virus had originated, an underlying question was how, and to what extent, it would mutate. To much relief, its mutation rate appeared to be modest at best — thanks in part to its proof-reading polymerase (Nsp14) that corrects errors during the genome replication process.1
As global cases quickly grew, SARS-CoV-2’s genome was given an ever-increasing opportunity to experiment. As early as March of 2020, sequencing had revealed a growing number of mutations in the virus’s spike gene, whose protein product is responsible for mediating cell-entry and pathogenesis. Of these, D614G (Aspartate 614 → Glycine) rapidly ascended to consensus, catching the attention of the life science and public health communities. Yet, as the mutation was found to confer only mild improvements to infectivity and shedding, attention soon returned to other aspects of SARS-CoV-2 biology.2
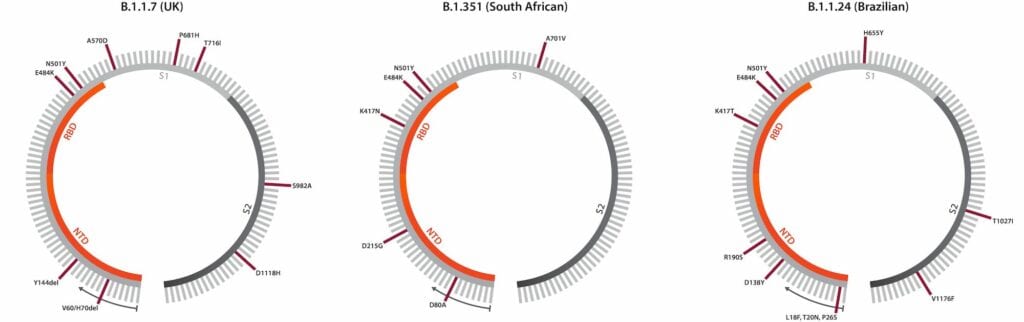
However, mutation still wasn’t a closed case. As SARS-CoV-2 infections soared through late 2020, the genomic landscape became significantly more diverse, culminating in December 2020, when three novel variants of concern (VoC) were all detected within the space of a month: B.1.1.7 in the UK, B.1.351 (501Y.V2) in South Africa, and B.1.1.24/P.1 in Brazil (Figure 1).
The many previous point mutations encountered made it clear that mutations were nothing new for SARS-CoV-2. However, what made the emergence of new variants so surprising was that each possessed a broader constellation of mutations not seen together before. Coinciding with their regional emergence, many countries also witnessed surges in case numbers, leading to the re-implementation of lockdowns and travel restrictions.3 Since late 2020, more VoCs have emerged, including the Californian B.1.429 and Indian B.1.617 variants.
Making Sense of the Mutations
For ongoing public health and vaccination programs, characterizing variants has become a clear priority. Beyond the immediate threat of increased transmissibility and virulence, concern has been growing around the robustness and longevity of human COVID-19 immunity: as all currently available vaccines target the original Wuhan-Hu-1 strain’s spike protein, significant-enough mutations could hypothetically enable variants to evade a substantial degree of immunity elicited by vaccines.
These fears were stoked in early 2021, when studies showed a reduced ability of patient sera to neutralize variants in vitro, and South African vaccine trials, where the B.1.351 variant was circulating, resulted in lower-than-expected protection.4,5 Kick-starting a new wave of research, considerable work has since been underway to characterize these variants and support the development of precautionary measures.
The constellation of Spike mutations that have emerged is substantial and ever-changing, with different substitutions, deletions and combinations having showed the ability to confer a range of different traits. Those that have gained the most attention so far, are K417N, E484K/Q, N501Y and L452R (Figure 2), all of which reside in Spike’s receptor-binding domain (RBD) — the immunodominant region responsible for ACE2 binding and the major target of neutralizing antibodies.6
These four mutational “hotspots” are all shared by the major B.1.1.7 (UK), B.1.351 (South African), B.1.1.24/P.1 (Brazilian), B.1.429 (Californian), and B.1.617 (Indian) variants. Having repeatedly arisen independently of one another, they appear to be advantageous low-hanging fruit for SARS-CoV-2 to adopt, alongside the many other, less significant mutations. These mutations have also shown to out-compete prototypic sequences, confer higher ACE2 affinity, and increase viral loads in patients.7 E484K, for example, has shown to confer up to 10-fold greater resistance to convalescent patient and vaccinee sera8, and evade binding of multiple RBD-specific monoclonal antibodies.9
A more recent VoC, B.1.617, has since emerged in India, including the E484Q and L452R mutations10, the latter of which has now converged in multiple variants.11 Like other point mutations, L452R has shown to increase infectivity and shedding, with small decreases to the neutralizing ability of anti-RBD antibodies.12 L452R may be of significant adaptive value to SARS-CoV-2, with recent containment measures and growing population immunity possible creating significant pressures for its positive selection.

Spike has also shown a tendency to lose small portions of its N-terminal domain. The H69/V70 deletion of the B.1.1.7 (UK) variant, for example, has shown to contribute to increased infectivity and PCR target failure.13 And finally, while recombination hasn’t gained significant attention outside of SARS-CoV-2’s putative origins and spill over, data suggests that it may be occurring and contributing to the emergence of novel variants to some degree.14Recombination events, through co-infecting variants in particular, may facilitate more rapid and drastic changes to the SARS-CoV-2 genome.
The Importance of Neutralization
The emergence of variants in recent months comes as a reminder that COVID-19 still has some surprises in store. As the majority of vaccines provoke an immune response to multiple spike epitopes, the breadth of antibodies elicited from vaccination are expected to continue conferring a moderate degree of protective efficacy and reduce the incidence of severe disease, at the least.15 However, the proportion of vaccine coverage needed to achieve herd immunity is unclear, and the bar may only rise higher if new variants show increased transmissibility in naïve and immune individuals. The emergence of variants may also be exacerbated by immune-mediated selection pressures from therapeutic-, patient- or vaccine-induced antibodies.16
A concerted global effort is now underway to track, characterize, and contain the novel SARS-CoV-2 mutations. Here, consensus and intra-host genome sequencing continues to play a fundamental role in variant surveillance17, generating a wealth of epidemiological data that can indicate whether mutations mediate infectivity, virulence, or immune evasion.
Deep mutational scanning and predictive simulations are also enabling prospective evaluations of mutation functionality to stay a few steps ahead.18 However, as we still lack a means of comprehensively and systematically assessing immunity at the patient level, significant gaps remain in our scientific arsenal.
Neutralizing antibody titers (Figure 3) appear to correlate well with the protective efficacy of vaccines, and so have largely defined correlates of protection.9 However, most large-scale serological studies to date have relied on rapid tests, which are unable to measure neutralizing efficacy, and are limited by relatively low sensitivity and specificity. In an attempt to address this gap, a new generation of immunoassays are being developed to enable more standardized means of assessing variant sensitivity to neutralization by monoclonal antibodies and vaccinee sera, as well as vaccine protection of animals against challenge with novel variants in research and preclinical testing.19
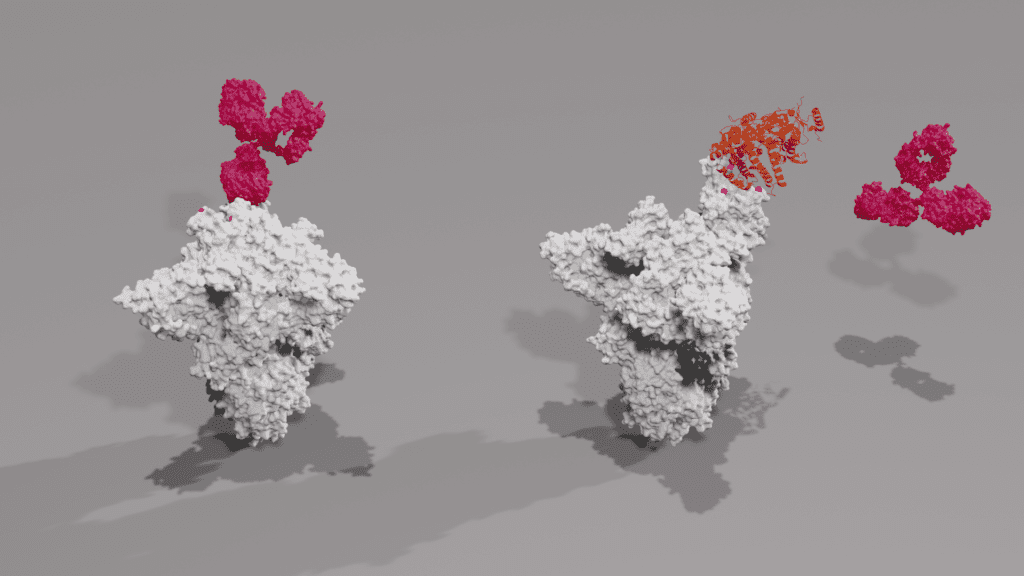
Figure 1: Schematics showing annotated spike gene sequences for the B.1.1.7, B.1.351 and B.1.1.24/P.1 variants, including both point mutations and deletions. Note that each lineage has shown to exhibit spike gene diversity, making these diagrams subject to change. Illustration: The Native Antigen Company
Figure 2: X-ray crystal structures of mutant spike trimer (grey) in closed conformation being bound by IgG antibody (dark pink). Key mutations are highlighted in pink and labelled accordingly. Photo: The Native Antigen Company
Figure 3: Principles of competitive binding neutralization assay. Above left: Antibody binds mutant spike trimer; Above right: Antibody fails to neutralize spike trimer, allowing ACE2 to bind RBD. Spike proteins are shown grey, point mutations are shown pink, antibodies are shown dark pink, ACE2 is shown orange. Photo: The Native Antigen Company
ABOUT THE AUTHOR
Andy Lane, PhD, is commercial director at The Native Antigen Company (now part of LGC’s Clinical Diagnostics business unit), a manufacturer of antigens and antibodies for infectious diseases, including SARS-CoV-2. Following his PhD in pathobiology, Lane joined the U.K.’s National Health Service (NHS) and led a monoclonal antibody research group working in leukemia and lymphoma diagnostics. He subsequently joined a major monoclonal antibody supplier to lead new product development and was executive director at Innova Biosciences.
REFERENCES
- Robson F, Khan KS, Le TK, et al. Coronavirus RNA Proofreading: Molecular Basis and Therapeutic Targeting. Molecular Cell. 2020;79(5), 710–727. https://doi.org/10.1016/j.molcel.2020.07.027.
- Volz E, Hill V, McCrone JT, Price A. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell. 2021;184(1), 64–75.e11. https://doi.org/10.1016/j.cell.2020.11.020.
- Priya P, & Shanker A. Coevolutionary forces shaping the fitness of SARS-CoV-2 spike glycoprotein against human receptor ACE2. Infection, Genetics and Evolution. 2021;87, 104646. https://doi.org/10.1016/j.meegid.2020.104646.
- Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. Novavax. 2021. https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3.
- Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use – First Single-Shot Vaccine in Fight Against Global Pandemic. Johnson & Johnson. 2021. https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic.
- Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855), 616–622. https://doi.org/10.1038/s41586-021-03324-6.
- Coutinho RM, Marquitti FMD, Ferreira LS, et al. Model-based estimation of transmissibility and reinfection of SARS-CoV-2 P.1 variant. MedRxiv. 2021. https://www.medrxiv.org/content/10.1101/2021.03.03.21252706v3.
- Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857), 130–135. https://doi.org/10.1038/s41586-021-03398-2.
- Collier DA, De Marco A, Ferreira I, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021;593(7857), 136–141. https://doi.org/10.1038/s41586-021-03412-7.
- SARS-CoV-2 variants of concern as of 24 May 2021. (2021). European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/covid-19/variants-concern.
- Tchesnokova V, Kulakesara H, Larson L, et al. Acquisition of the L452R mutation in the ACE2-binding interface of Spike protein triggers recent massive expansion of SARS-Cov-2 variants. BioRxiv. 2021;2021.02.22.432189.https://doi.org/10.1101/2021.02.22.432189.
- Deng X, Garcia-Knight MA, Khalid MM, et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021;S0092-8674(21)00505-5. Advance online publication. https://doi.org/10.1016/j.cell.2021.04.025.
- Brown KA, Gubbay J, Hopkins J, et al. S-Gene Target Failure as a Marker of Variant B.1.1.7 Among SARS-CoV-2 Isolates in the Greater Toronto Area, December 2020 to March 2021. JAMA. 2021; e215607. Advance online publication. https://doi.org/10.1001/jama.2021.5607.
- Ignatieva A, Hein J & Jenkins, PA. Evidence of ongoing recombination in SARS-CoV-2 through genealogical reconstruction. BioRxiv. 2021. https://www.biorxiv.org/content/10.1101/2021.01.21.427579v1.
- Liu Z, VanBlargan LA, Bloyet LM, et al. Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. BioRxiv. 2020;2020.11.06.372037. https://doi.org/10.1101/2020.11.06.372037.
- Rees-Spear C, Muir L, Griffith SA, et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Reports. 2021;34(12), 108890. https://doi.org/10.1016/j.celrep.2021.108890.
- Valesano AL, Rumfelt KE, Dimcheff DE, et al. Temporal dynamics of SARS-CoV-2 mutation accumulation within and across infected hosts. PLoS pathogens. 2021;17(4), e1009499. https://doi.org/10.1371/journal.ppat.1009499.
- Starr TN, Greaney AJ, Hilton SK, et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell. 2020;182(5), 1295–1310.e20. https://doi.org/10.1016/j.cell.2020.08.012.
- Mascola, JR, Graham BS, & Fauci AS. SARS-CoV-2 Viral Variants-Tackling a Moving Target. JAMA,2021;325(13), 1261–1262. https://doi.org/10.1001/jama.2021.2088.