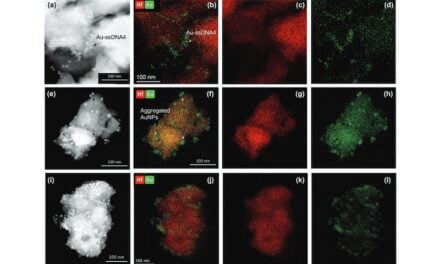
The collaboration aims to create a non-invasive diagnostic for Pseudomonas aeruginosa using miniaturized gas chromatography-mass spectrometry technology.
Detect-ION LLC has launched a collaborative project with the Infectious Diseases Division at Mayo Clinic Florida to develop a breath-based diagnostic test for detecting Pseudomonas aeruginosa, a leading cause of pneumonia in lung transplant recipients and patients with structural lung diseases.
The project, funded through the Mayo Clinic Advanced Innovation Research program, seeks to address diagnostic challenges posed by P. aeruginosa, which causes hospital-acquired pneumonia and ventilator-associated pneumonia cases that can lead to sepsis or respiratory failure. The bacterium’s ability to form protective biofilms and resist antibiotics makes it difficult to treat, contributing to broad-spectrum antibiotic overuse and drug-resistant strain development.
Current diagnostic methods including sputum and blood cultures are often inconclusive, while bronchoscopy is costly, invasive, and unsafe for many high-risk patients. These methods frequently fail to distinguish between invasive strains and colonizing strains that are unlikely to cause infections, resulting in overtreatment with antibiotics.
CLARION Technology Platform
The project will use Detect-ION’s CLARION platform, a point-of-care breath diagnostic system powered by a patented miniaturized gas chromatograph and chip-scale mass spectrometer. The system delivers laboratory-grade analysis in under five minutes, detecting trace volatile organic compounds in exhaled breath.
After an initial biomarker discovery phase using high-resolution mass spectrometry, researchers aim to identify biomarkers in exhaled breath that can distinguish between invasive and colonizing P. aeruginosa strains.
CLARION was originally developed as dual-use technology for trace chemical sensing through funding from US government agencies including IARPA, DARPA, DTRA, and DIU. The platform is now being advanced for clinical applications including pulmonary infections, malaria, tuberculosis, and lung cancer.
Addressing Healthcare Costs
In the United States, pneumonia drives more than $17 billion in annual healthcare costs and often leads to prolonged hospital stays. The collaboration aims to develop a scalable, non-invasive breath test that will allow physicians to intervene earlier, tailor treatments more precisely, and use systemic antibiotics more judiciously while expanding preventive strategies such as inhaled antibiotics.
“For decades, the gold standard of chemical analysis has been limited to laboratory settings,” says Dr Ashish Chaudhary, chief executive officer of Detect-ION, in a release. “With CLARION, we’ve miniaturized this capability and adapted for point-of-care breath analysis that now detects diseases in minutes. This collaboration allows us to apply this technology to one of medicine’s biggest challenges: diagnosing pneumonia quickly and accurately.”
Founded in 2021, Tampa-based Detect-ION develops molecular diagnostics through advanced sensor technologies with applications spanning national security, public health, and environmental monitoring.
ID 27459194 © Ginasanders | Dreamstime.com