By Thomas Schmidt, PhD
The covid-19 pandemic has vastly increased the demand on clinical laboratories’ testing capacity while simultaneously imposing restrictions to protect the health of the staff. In times like these, tools such as laboratory information management systems and electronic laboratory notebooks can not only streamline operations, they can also help meet emergency needs.
Scientific lab operations are quite complex, and no two labs are the same. Factors that can contribute to the complexity include the type of research; the variability of sample types, lab procedures, and software packages; the data produced; as well as the various types of instrumentation and consumables; and, most important, the lab personnel. An increasingly complex lab environment may also have developed as lab systems grew and were integrated with business systems and other lab informatics tools.

The challenge many lab managers now face is how to optimize lab operations in such a complex environment. Some challenges may be perceived as onerous—for example, ensuring that lab operations are compliant with the many and various regulations. But by attending to these requirements of reviewing and updating lab protocols, processes, and workflows, many labs come out the other end with a positive effect. With more technical controls and increased automation, labs experience improved turnaround time and higher quality results.
Incorporating more technical controls offers great potential to move toward a paperless lab. Discussions on this topic abound, but actual progress toward paperless labs has been moderate at best. Why? Going paperless in the lab does not mean just having electronic versions of worksheets or test execution protocols—It is also about the business intelligence that connects the electronic protocol with the lab instruments as well as the chemical inventories to assess the status of the assets. It is the communication with balances, including other small lab devices and the data systems set up to provide the status and location of samples and the corresponding research at any time.
To date, there has been no turnkey or standard solution for labs because there is no “standard” lab. What is required is a solution that can manage the complexity and heterogeneity of any particular lab. The challenge is to map the complexities within that lab and then provide a solution that shields these complexities from the user—a solution that can manage and track all analysis requests and samples, will help execute workflows in a way that errors are inherently eliminated and will also help manage the data in the lab, in an optimal way, and store it in a central location.
A big question for many lab managers is what kind of system(s) do they really need. There are three general types of tool:
- Laboratory information management systems (LIMS) are business applications that manage and track analysis requests and the associated samples, test results, and other information required to issue the final certificate of analysis (COA). Some LIMS provide direct interfaces to chromatography data systems (CDS) to mitigate the risk of errors due to the repeated manual transcriptions of entering sample information. A LIMS interface to the CDS makes good sense since most labs have documented their procedure execution, especially the sample preparation and observations on paper.
- Laboratory information systems (LIS) are individual-based, as opposed to LIMS which work better for groups of records that need to be processed at once. An LIS stores and manages an individual’s data and results, including features such as: tracking, storing, and managing an individual’s demographics and associated sample information; managing tests performed on samples and their outcomes; and generating individual compliant reports. For example, LIS may be installed in a hospital lab, whereas LIMS are typically deployed in industry laboratories.
- Analytical electronic laboratory notebooks (ELN), also sometimes referred to as laboratory execution systems (LES), provide the means to guide users electronically through their procedures. This does, of course, require the conversion of the paper protocols into electronic workflows, which in most cases can be quite a laborious task.
The lines between all these different lab tools have increasingly blurred over the last few years with established LIMS vendors adding ELNs to their portfolio, and ELN vendors adding sample tracking and management capabilities to their products.
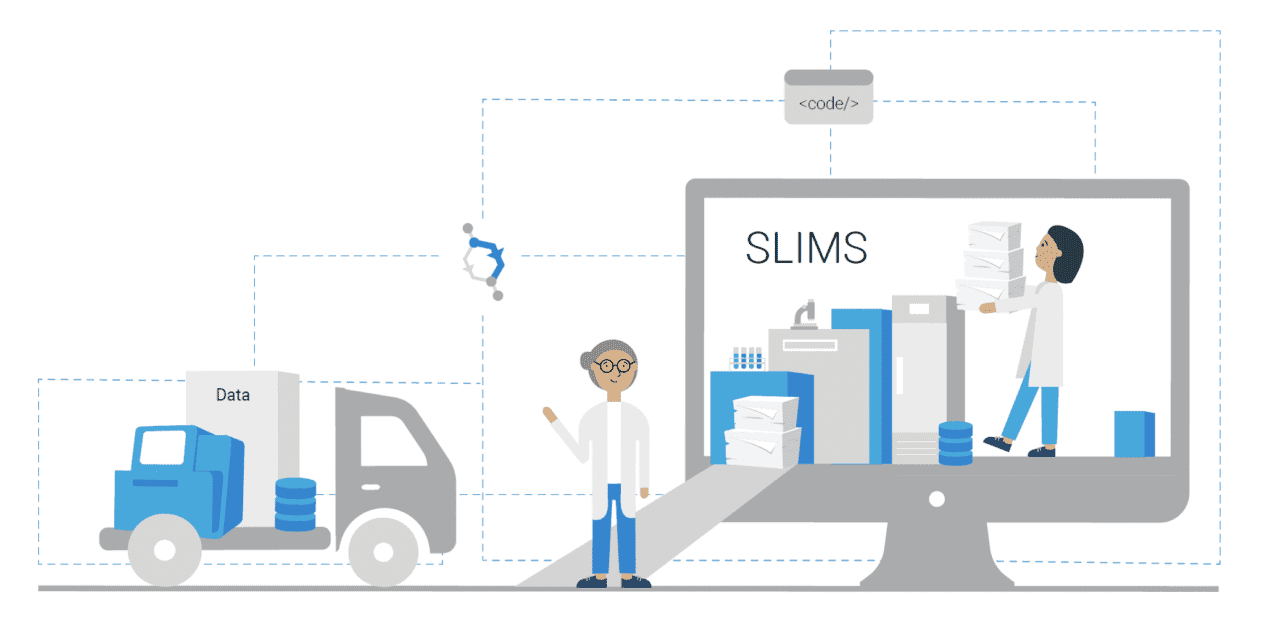
Alternative offerings have since been developed, with sample management and workflow execution in one software solution. An example is SLIMS from Agilent, an integrated and complete browser-based solution that encapsulates the primary components from both LIMS and ELN that are required for lab personnel to manage their lab operations. With downloadable workflow modules, SLIMS enables labs to customize a configuration to match their lab processes and procedures, vastly shortening project implementation times, so labs become productive in weeks, not months. Also, the system is designed to support the requirements of ISO17025, 21 CFR Part 11, HIPAA, and CLIA.
As the debate continues regarding the use of a single vendor platform versus best-of-breed applications with a multivendor solution, there is no doubt that as labs move increasingly toward a standardized and regulated environment, a single platform is an interesting and enticing option to consider. Reducing the time to realize the monetary benefits of automation, while minimizing workflow disruption, makes it worthwhile to look at SLIMS as an optimal solution for any lab.
Thomas Schmidt, PhD, is marketing director of the software and informatics division at Agilent Technologies, Santa Clara, Calif.
Featured image: SLIMS can be applied within various fields to facilitate data collection and record keeping, improving reproducibility and compliance, and streamline processes.