Verichem Laboratories’ new liquid stable and ready-to-use Matrix Plus Chemistry Reference Materials are designed for the calibration and calibration verification of wet chemistry assays on clinical instrument systems.
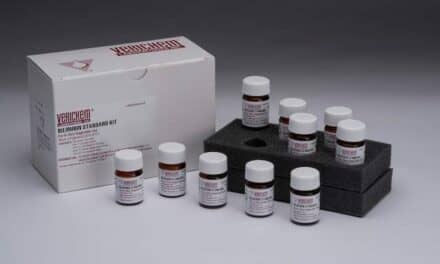
The multi-level Matrix Plus Chemistry Reference Materials Kit, along with the optional and stand-alone Level F, are intended to support overall system QC and CLIA compliance.
The products serve as tools in determining a clinical system’s bias to the true, or known, value and are certain to meet the needs of a variety of laboratory professionals involved in routine clinical testing, research applications, or with in-vitro diagnostic (IVD) product manufacturing. This multi-analyte and comprehensive six–level set of liquid stable materials contains seven individual chemistry components, covering a total of 48 separate concentration levels in total. Available analytes include Blood Urea Nitrogen (BUN), Calcium, Creatinine, Glucose, Magnesium (in mg/dL and mEq/L), Phosphorus, and Triglyceride and is suitable for use on multiple analyzers utilizing a wide variety of testing methods, including visible spectrum, ultraviolet, kinetic, and/or endpoint.
Verichem’s Matrix Plus Chemistry Reference Materials Kit incorporate a buffered protein based matrix for serum-like reactivity and is intended to be treated just as a patient specimen with clinical testing systems. The formulation displays remarkable optical clarity and is free of glycols, surfactants, azide and other interfering substances. By maintaining a constant protein content and neutral pH across concentration levels, the unique formulation and CLSI EP06-A set point design of these reference materials are crucial for the independent determination of test method accuracy, sensitivity, linearity and reportable range.
Plus, the use of highly purified source components and the liquid stable format eliminates matrix variations and reconstitution errors common experienced with lyophilized, or other serum-based products. Each active component is verified using available Standard Reference Materials from the National Institute of Standards and Technology (NIST) and fully documented with a Certificate of Analysis. Additionally, the Matrix Plus Chemistry Reference Materials Kit are packaged for multi-use and contain fill volumes ample enough to cover multiple verification testing events. Each of the levels are filled within opaque polyethylene dropper vials and feature open-vial product stability claim of 21 months from date of manufacture.
For more information, visit www.verichemlabs.com.
Featured image: The multi-level Matrix Plus Chemistry Reference Materials Kit, along with the optional and stand-alone Level F, are intended to support overall system QC and CLIA compliance. Photo: Verichem Laboratories