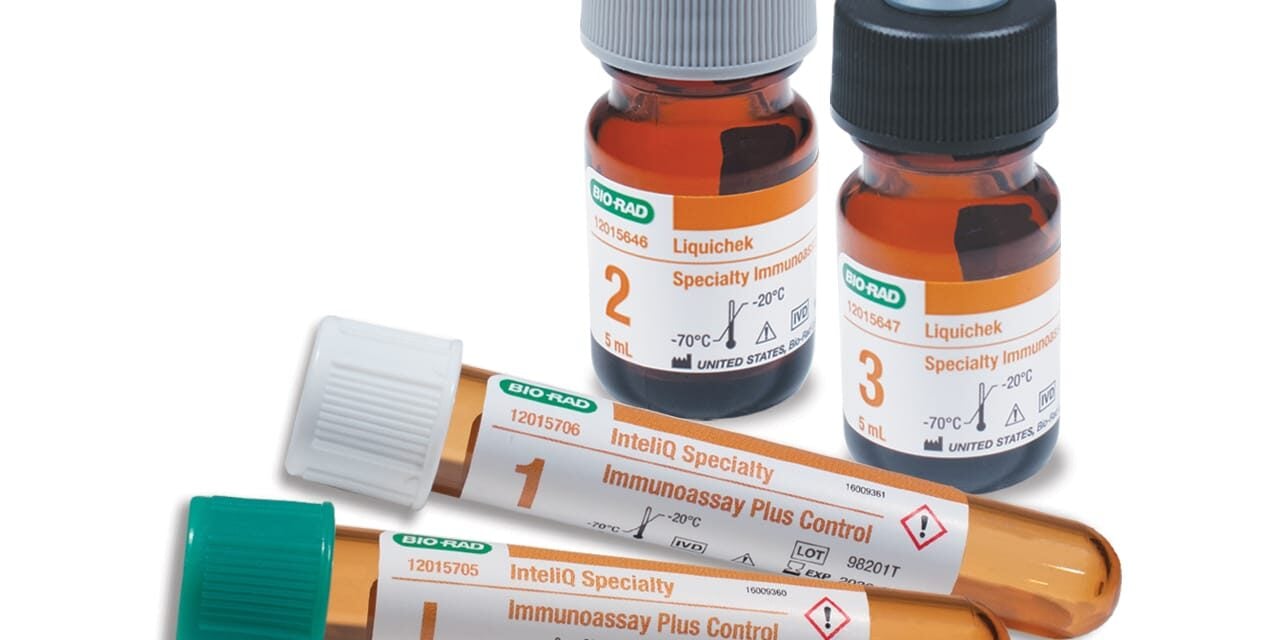
The four-level quality control product features procalcitonin in liquid format and automation-compatible configurations.
Bio-Rad Laboratories has launched its Specialty Immunoassay Plus (SPIA Plus) quality control product in Europe, offering laboratories a consolidated, liquid specialty immunoassay control system.
The four-level quality control is designed to monitor the precision of laboratory specialty immunoassay testing procedures. SPIA Plus features clinically relevant analytes including procalcitonin, IL-6, active vitamin B12, TRAb, TSI, and fructosamine.
The product is available in barcoded, load-and-go InteliQ and standard vial Liquichek configurations. Bio-Rad designed the system with optimized vitamin D ranges to support Vitamin D Standardization Program-compliant assays and improved intact PTH open-vial stability to minimize quality control waste.
Automation-Compatible Design
SPIA Plus offers automation-compatible configurations intended to enable load-and-go efficiency. The InteliQ format aims to streamline laboratory workflows to save time and resources.
The system integrates with Unity data management software, allowing laboratories to compare results across Bio-Rad’s peer group of reporting labs for instrument performance monitoring and patient result confidence.
Bio-Rad positions SPIA Plus as the next generation of independent specialty immunoassay quality controls, building on the company’s existing quality control portfolio for clinical laboratories.
The product is available now to laboratories throughout Europe.