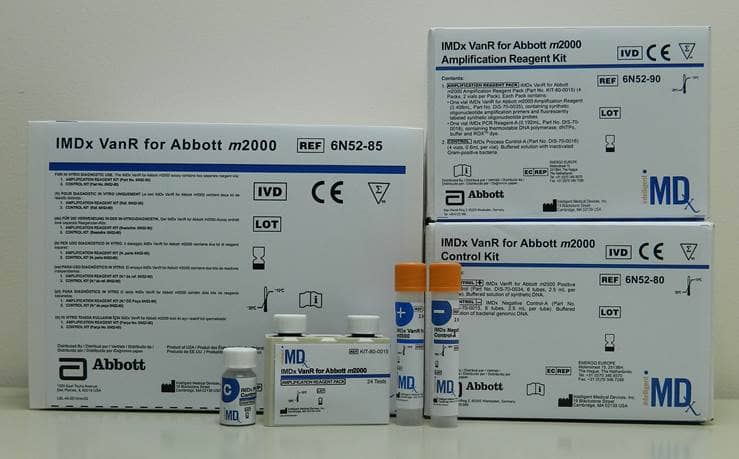
This test is among those in the company’s portfolio of infectious disease products to be cleared for use on Abbott’s fully automated m2000 platform.
The assay is intended to be used as an aid to identify, prevent, and control vancomycin-resistant colonization in health care settings.
The automated nature of this test allows labs to obtain results for up to 46 patient samples in less than 3 hours, and up to 94 patient samples in less than 4 hours.
The assay is performed directly on human peri-rectal swabs, rectal swabs, or stool specimens from patients at risk for VRE colonization.
[Source: Abbott]