Study supports a potential technology antidote to difficult blood draws and failed IV starts
By Andrew Barton, BSc
For most clinical laboratories blood analysis is a key function. However, obtaining a sample for analysis is not always easy. Blood draws from children are generally difficult, and a wide range of factors such as chronic disease, diabetes, obesity, old age, renal disease, and vascular pathology can complicate adult blood draws.
Failed blood draws, or samples that are unsuitable as a result of a difficult draw, can lead to increased cost and delayed treatment. A 2002 Q-Probes study of outpatient phlebotomy found that 13% of unsuccessful phlebotomies were the result of difficult draws.1 A further 11.8% of failed phlebotomies were the result of patients leaving without blood being collected. It was not clear how many patients required multiple attempts or left because a sample could not be obtained. But when a sample was obtained and subsequently rejected, the reasons cited for the failure included insufficient volume or clotting.
First-Stick Failures
Over the years, a moderate amount of research has been done on first-stick failure rates for intravenous line (IV) starts. First-stick failure rates for IV starts are generally quite high, at 26% for adults and 47% for children.2–4 This figure represents the frequent need for multiple attempts, in both adults and children, prior to gaining successful insertion of an intravenous cannula.
Much less research has been done on first-stick failure rates for blood draws. Typically, documentation is not collected on the number of attempts prior to attaining a successful blood sample. According to the 2002 Q-Probes study, most laboratories have a policy of permitting no more than four attempted blood draws per patient. Such policies are in line with guidelines of the Infusion Nurses Society for the placement of IV cannulas, which stipulate that there should be no more than 2 attempts per clinician, and no more than four total attempts per patient.5
For the patient, multiple venepuncture attempts are an unpleasant and often painful experience that results in vein depletion.6 Vascular access nurses and phlebotomists have long considered the application of heat—usually in the form of a heat pack placed over the target vessel—to be an effective way of dilating vessels, aiding in vein identification and improving first-stick success.7
A Technology Alternative
As is common in medicine, whenever a practice is successful, it becomes a target for technology improvements by physician-inventors and product developers. So it should not be surprising that a new product called Veinplicity, from medical device manufacturer Physeon, Schaffhausen, Switzerland, has recently been developed as an enhanced alternative to the age-old heat pack standard of care to dilate veins and improve successful blood sampling and intravenous cannulation (Figure 1).
Physeon is a boutique medical device company established in 2015 to guide the development and commercialization of innovations in healthcare. The company embraces science and research to explore innovative ideas and develop medical products that can advance the health and well-being of patients and simplify processes for healthcare professionals.

Figure 2. Ultrasound images of vein diameter measurements. Vein diameter at baseline (left) and after stimulation with Veinplicity (right). The white dot represents a 22°G cannula occupying the vein’s cross-sectional area before treatment (11% overlap) and after treatment (3% overlap).
Veinplicity consists of a portable electronic device that passes a gentle current between paired electrodes placed on the patient’s palm and biceps. The resulting electrical stimulation is designed to increase the size and tone of forearm vessels, making them easier to palpate and access for both IV starts and blood draws.
The Veinplicity device is CE marked and is available in the EU. Although Physeon is currently seeking FDA marketing authorization for the use of Veinplicity in IV cannulation, the device is not yet authorized by FDA and is not for sale in the United States.
At Frimley Park Hospital, it is our policy to use appropriate technology to minimize failed IV sticks. In this way we help to preserve precious vessels, improve sample integrity, and reduce waste. We also reduce treatment delays, minimize discomfort, and increase patient satisfaction.
With these goals in mind, we undertook a study to compare the dilatory effects of the Veinplicity device to the effects of the heat packs that have traditionally been used to dilate target vessels. For both approaches, we used ultrasound to measure the mean increase in vein diameter (Figure 2).

Table 1. Baseline vein diameter for participants in a study comparing the dilatory effects of the Veinplicity device to the effects of heat packs.
Study Findings
To perform our study, we randomized 20 volunteers to receive either application of heat packs to the forearm and later stimulation with Veinplicity, or the same two treatments in reverse order (Table 1). Ultrasound measurements of the basilic, cephalic, and brachial veins were taken at intervals during and after treatment, and the measurements were compared with baseline values (Figure 3).
The study found that the increase in mean maximum vein diameter over baseline was significantly higher with Veinplicity than with heat packs (49.94% ± 23.55% versus 36.26% ± 23.09%; p = 0.021). In addition, the mean duration of the dilatory effect was significantly longer with Veinplicity than with heat packs (9.7 ± 3.9 minutes versus 4.9 ± 2.2 minutes; 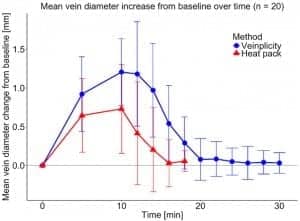
For study participants with a higher body mass index, electrical stimulation was found to be less effective at vessel dilation, but still more effective than heat treatment. Nevertheless, we did not further investigate the statistical significance of this variable and recognize some interventions may require a multimodal approach.
The study demonstrated that the Veinplicity device dilates arm veins more effectively and for a longer period than the traditional heat pack standard of care (Figure 4). In practice, it has also been observed that Veinplicity gives better tone to target vessels, making them easier to identify in the first place, and less likely to roll away when the phlebotomist is attempting to insert a needle.

Figure 4. Strip plots of the maximum mean percentage vein diameter change (left) and mean treatment effect duration (right). The calculated averages of the means are shown in red, including standard deviations.
Conclusion
At Frimley Park Hospital, we have not yet attempted to measure the effects of using the Veinplicity device on first-stick success. However, a recent study involving a cohort of preoperative patients at Catharina Hospital found significantly improved first-stick cannulation success when Veinplicity was used prior to tourniquet, as compared to the use of a tourniquet alone.8
As healthcare providers treat more and more acute illness and an aging population, venous access challenges are inevitably increasing. It is important that providers do whatever they can to promote vessel health and preservation and improve first-stick success rates—including the appropriate use of proven technologies. Adopting the new Veinplicity technology would support those aims and be a step in the right direction to preserve veins and improve efficiency, as the device is easy for practitioners to use, and has the potential to spare patients the discomfort, pain, and even trauma that come with multiple access attempts.
Andrew Barton, BSc, is an advanced nurse practitioner for IV therapy and vascular access at Frimley Health NHS Foundation Trust. For further information, contact CLP chief editor Steve Halasey via [email protected].
References
- Dale JC, Novis DA. Outpatient phlebotomy success and reasons for specimen rejection. Arch Pathol Lab Med. 2002;126(4):416–419; doi: 10.1043/0003-9985(2002)1262.0.co;2.
- Carr PJ, Rippey JC, Budgeon CA, Cooke ML, Higgins N, Rickard CM. Insertion of peripheral intravenous cannulae in the emergency department: factors associated with first-time insertion success. J Vasc Access. 2016;17(2):182–190; doi: 10.5301/jva.5000487.
- Lapostolle F, Catineau J, Garrigue B, et al. Prospective evaluation of peripheral venous access difficulty in emergency care. Intensive Care Med. 2007;33(8):1452–1457; doi: 10.1007/s00134-007-0634-y.
- Lininger RA. Pediatric peripheral IV insertion success rates. Pediatr Nurs. 2003;29(5):351–354.
- Policies and Procedures for Infusion Therapy. 5th ed. Norwood, Mass: Infusion Nurses Society, 2016. Available at: http://ins.tizrapublisher.com/hha7v4/76. Accessed July 31, 2019.
- Hawes ML. A proactive approach to combating venous depletion in the hospital setting. J Infus Nurs. 2007;30(1):33–44.
- Hallam C, Weston V, Denton A, et al. Development of the UK vessel health and preservation (VHP) framework: a multiorganizational collaborative. J Infect Prev. 2016;17(2):65–72; doi: 10.1177/1757177415624752.
- Van Loon FH, Willekens FJ, Buise MP, Korsten HH, Bouwman AR, Dierick-van Daele AT. Clinical use of electrical stimulation with the Veinplicity device and its effect on the first-attempt success rate of peripheral intravenous cannulation: a non-randomized clinical trial. J Vasc Access. Epub ahead of print, March 28, 2019; doi: 10.1177/1129729819838093.







