New platforms provide standardized histopathology endpoints for drug developers on company’s AISight Clinical Trials Platform.
PathAI expanded its AISight Clinical Trials Platform with the launch of IBDExplore to join AIM-HI UC, providing drug developers with AI-powered tools for inflammatory bowel disease clinical trials.
The Boston-based AI pathology company announced the launch makes these solutions immediately available for integration into clinical trial protocols as standardized, reproducible endpoints. Both tools were developed in partnership with the Foundation for National Institutes of Health (FNIH) Biomarkers Consortium.
The AISight Clinical Trials Platform provides a GCP/GCLP-compliant environment that bridges traditional pathology and computational precision, according to PathAI.
IBDExplore Provides Spatial Analysis
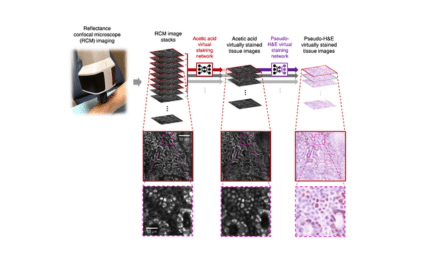
While AIM-HI UC provides standardized, reproducible histologic scoring, IBDExplore converts standard H&E-stained histology whole slide images into quantitative spatial insights. When integrated into clinical trial outputs as an exploratory endpoint, IBDExplore enables researchers to analyze tissue architecture and cellular patterns.
“The integration of AIM-HI UC and IBDExplore into the clinical trial landscape represents a paradigm shift in how we evaluate treatment efficacy and directly addresses the FNIH project team’s aim to bring standardization to mucosal healing assessment,” says Stephen Laroux, director – scientific research fellow, precision medicine & immunology at AbbVie and co-chair of the FNIH Mucosal Healing in UC project team, in a release.
“By leveraging AI to capture subtle histological nuances and spatial patterns that often go undetected by the human eye, we can achieve a more precise and objective understanding of mucosal healing,” Laroux says in a release.
Multi-Company Collaboration
The FNIH Biomarkers Consortium Mucosal Healing in Ulcerative Colitis project team includes eight life sciences companies, non-profit foundations, academic medical centers, key opinion leaders, and FDA regulatory representatives. The consortium leads cross-sector efforts to validate and qualify biomarkers that accelerate therapeutic development.
“The launch of both IBDExplore and AIM-HI UC on the AISight Clinical Trials platform transforms how we measure success in ulcerative colitis trials,” says Andy Beck, CEO at PathAI, in a release. “By moving toward deeper insights and automated, reproducible histology, we aren’t just improving data quality, we are accelerating the delivery of life-changing treatments to patients.”
PathAI notes that IBDExplore, AIM-HI UC, and the AISight Clinical Trials Platform are for research use only and not for use in diagnostic procedures.
The company provides AI-powered pathology solutions aimed at improving histology assessment accuracy and accelerating drug development. PathAI’s platform uses artificial intelligence to analyze and interpret pathology images for pathologists, researchers, and pharmaceutical companies.
ID 130758328 © Ivan Shidlovski | Dreamstime.com