Zymo Research announced that the U.S. Food and Drug Administration (FDA) cleared its DNA/RNA Shield SafeCollect Saliva Collection Kit as a Class II medical device for microbial nucleic acid storage and stabilization.
The FDA’s 510(k) clearance for Zymo allows the product to be used as an In-vitro Diagnostic (IVD) device for saliva collection and transport.
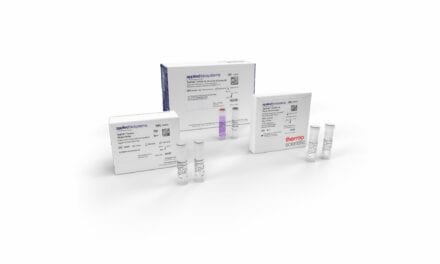
Specifically granted for COVID-19 testing, the SafeCollect Saliva Collection Kit utilizes DNA/RNA Shield, an FDA-cleared inactivating transport medium (ITM) to demonstrate inactivation and preservation of the SARS-CoV-2 RNA virus. The properties of the ITM simplify handling, transportation, and storage of the specimen while also maintaining the integrity of viral RNA at ambient temperature for extended periods, ensuring robust downstream analysis via RT-PCR.
“Non-invasive saliva collection is vital for the molecular diagnostics field, unlocking a wealth of genetic and biomolecular information with ease and comfort, paving the way for groundbreaking advancements in personalized healthcare and disease detection” says Stanislav Forman, PhD, CTO of Sample Prep at Zymo Research. “This 510(k) clearance highlights the FDA’s initiative in bringing enabling technologies to the maturing molecular diagnostic space.”
Further reading: FDA Clears OTC COVID Home Antigen Test
The product features a user-friendly spit funnel with an innovative safety seal designed to prevent accidental spillage or user exposure to the DNA/RNA Shield preservative reagent in the tube. The technology is compatible with human saliva suspected of containing SARS-CoV-2. Specimens collected and stored are suitable for use with appropriate molecular diagnostic tests.