There is a brisk competition for expanding market.
According to the American Diabetes Association, 20.8 million children and adults, or 7% of Americans, have diabetes mellitus. And the number is rising. The global projection for the year 2025 — 333 million — is larger than the current US population.
Increased rates of obesity combined with a trend toward more sedentary lifestyles assure the continual increase of an as yet incurable disease with many potentially devastating complications.
Widespread elevation in impaired fasting glucose levels testifies to the growing danger, and the consensus among health professionals is that there is, indeed, an ongoing diabetes epidemic.
Diabetes places a tremendous physical, emotional, and economic burden on the individual, and on society as a whole. With the disease costing an estimated $135 billion per year in the United States alone, early diagnosis and accurate monitoring have become increasingly important.
Because diabetes is an illness that can be only forestalled or managed, companies involved in the development of testing devices are engaged in brisk competition for this expanding market, both in clinical labs and particularly in newer and fast-growing point-of-care (POC) testing technology.
Diabetes testing devices take a variety of forms due to the different testing approaches, such as glucose tolerance, HbA1C, and albumin, and within hemoglobin testing, the differing methods of HPLC, immunoassay, and the more recently developed enzymatic. Within each category, various factors present challenges calling for innovative technology to improve patient care, cost, and efficiency.
Direct Enzymatic Approach
At Diazyme Laboratories, San Diego, the quest is to optimize the HbA1c testing methodology by using what the company calls a “direct enzymatic” approach. The objective is to create an accurate, simple, and cost-effective procedure that includes benefits such as single-channel usage, elimination of hemoglobin variants, and correlation with HPLC and immunoassays.
“Most currently marketed HbA1c assays use a dual-channel format,” says Doug Borses, Diazyme’s marketing manager. “Our newly developed direct enzymatic technology, which is applicable to most clinical analyzers, eliminates the need for a second channel by directly measuring the %HbA1c, and therefore is more cost-effective.”
Another key benefit of the technology, according to Borses, is the virtual elimination of interference from hemoglobin variants. Testing is not adversely affected by Hbs, C and E variants in samples. Additionally, the system uses reagents that don’t contain latex particles, thus eliminating contamination of analyzer cuvettes and lines, which reduces maintenance time and costs for laboratories.
Diazyme’s new assay, recently approved by the FDA, is a clinical laboratory procedure. However, in keeping with what Borses calls the “trend toward convergence,” Diazyme is also developing a POC version expected to launch in 2008. The Smart 700 analyzer, for use in physicians’ offices, will measure 9.4 inches x 5.5 inches x 5.7 inches. The analyzer will provide advantages similar to the clinical lab version, such as low test cost, high reference correlation, ease of use, and low maintenance.
Intuitive Testing
Development in POC diabetes testing is also under way at BioRad Laboratories, Hercules, Calif. BioRad’s new assay, in2it, is a fully automated system designed for simplicity, convenience, and speed in near-patient testing. It requires 10uL of capillary or venous blood while using boronate affinity chromatography for results traceable to the worldwide DCCT reference. As its name suggests, “it’s intuitive,” says Tamara Davis, marketing manager.
The assay is CLIA waived and NGSP certified, supports multilanguage screen displays, and offers computer connectivity for complete data management. Scheduled for launch in the fall, the product will be eligible for the new CPT code.
BioRad also offers advanced technology for laboratory testing. In collaboration with Sysmex, an automated hematology and HbA1c testing solution called the Variant II Turbo Link, which integrates HbA1c testing into Sysmex’s HST-N hematology automation platform, has been developed.
The improvement in well-established chemistry, says Davis, provides efficient sample handling through workstation consolidation, and assists patient care by exposing potential variant hemoglobins. Turnaround time is also decreased through the use of Symex’s Molis Wam software.
QUANTITATIVE TESTS
The people at Siemens offer the new Vantage analyzer as the company’s entry into the POC testing market. “Our DCA platforms have done well in clinically relevant quantitative tests for diabetes management,” reports Senior Vice President Arnd Kaldowski. The HbA1c test, says Kaldowski, was used in the key clinical papers that were pivotal in establishing this test as the standard of practice for helping physicians drive glycemic control at the point of care. In addition to the HbA1c tests, the DCA platforms perform microalbumin creatinine testing, including albumin-to-creatinine ratio testing for detection of early kidney disease, a major complication of diabetes. The company also offers urinalysis strip products for detection of diabetes and for detection of complications of diabetes in testing with its Multistix test strips and Clinitek POC analyzers.
“The DCA platform offers precise and accurate HbA1c results at the point of care, which has been proven in over 100 clinical papers and continues to hold true in external proficiency studies,” Kaldowski says.
The system is a POC testing device with no sample or reagent handling preparation steps, and the smallest whole blood capillary sample of 1uL that is accurately obtained and not technique sensitive.
New features include an onboard printed patient record, HbA1C trending graphs, and a database for improved patient management. DCA tests are reimbursable at the point of care.
Siemens maintains a comprehensive product offering for the clinical laboratory, including immunoassay, clinical chemistry, hematology, molecular, and various other tests. For diabetes management, glucose and HbA1c tests are menu items on the Advia chemistry analyzers and the Immulite products.
Multiwell Technology
Nova Biomedical, Waltham, Mass, saw a need in the market for bedside glucose testing in hospitals. The single-well POC measurement technology in use for the past decade was originally developed for self-testing at home with little consideration for the increased acuity of hospital patients. Therefore, many errors are commonly found in hospital usage, including significant interferences, large biases, and poor correlation with central lab reference methods.1-4 The home test strips, according to Nova, give inaccurate results among patients with abnormal hematocrit levels, patients on hemoglobin therapy or peritoneal dialysis, and patients with high or low blood oxygen. These limitations are further compounded by new protocols such as tight glycemic control, which has been shown in studies to reduce morbidity and mortality for critical care and surgical patients by 30% or more.5-8
To meet the more demanding requirements of hospital glucose testing, and to achieve the newly required lower glucose levels without the risk of hypoglycemia, Nova has developed a multiwell technology, StatStrip. It eliminates interferences such as acetaminophen, uric acid, and ascorbic acid, all of which compromise glucose testing results. The product also eliminates interferences from maltose and glactose present in immunoglobulin preparations. Interference of oxygen and icodextranis—present in dialysis solution—is also eliminated. The new four-well system has a 6-second analysis time and a simple, color touch screen operation. StatStrip requires no calibration codes, thus eliminating an operator step and preventing a potential input error.
Immunoassays
Kronus, Inc, Boise, Idaho, offers more than a dozen immunoassay test kits and related laboratory reagents for the diagnosis and management of various autoimmune and endocrine/metabolic disorders, including diabetes. Specializing in Type 1 diabetes, Kronus addresses the need for specific immune-related markers to help those who are at the greatest risk of developing diabetes.
-
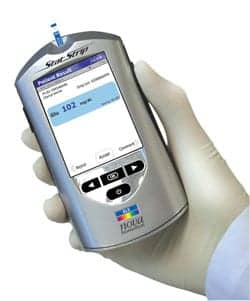
- Nova Biomedical Stat Strip
Before clinical onset of the disease, Type 1 diabetes is generally characterized by lymphocytic infiltration of the islet cells and circulating antibodies against a variety of islet cell antigens, including glutamic acid decarboxylase (GAD) and IA-2. However, sufficient insulin deficiency leading to diabetes does not usually occur until 80% to 90% of the insulin-secreting beta cells have been destroyed, thus providing a prolonged prediabetic stage in which these disease markers are present and measurable. Because it can be several years before the clinical onset of the disease, the ability to accurately identify its etiology before clinical presentation can enhance early and aggressive intervention and overall disease management.
That’s when Kronus’ ELISA and RIA immunoassay test kits come to the fore. Because there is no cure for diabetes, early diagnosis is crucial. By identifying and measuring the autoantibodies GAD and IA-2, which are found, respectively, in 70% to 90% and 50% to 75% of Type 1 diabetic patients, improved quality of life for the individual as well as overall reduction in medical expenses are possible.
The measurements provided through use of the ELISA and RIA test kits have also been shown to be of significant value in diagnosis, management, and prediction of development of diabetes in first-degree relatives of diabetic patients.
Reproducibility
Tosoh Bioscience, San Francisco, offers its High Performance Liquid Chromotography (HPLC) method of glycated hemoglobin testing, which is distinctive, says product manager Jane Merschen, because of its reproducibility, or precision. “The HPLC method excels with less than 1% CV,” she says.
-
- Tosoh G8
Tosoh’s new G8 analyzer, which is nearing FDA approval, will have an elution time of 1.6 minutes and will use cation exchange protein separation technology. It will improve upon the current G7 version both in elution time (the G7 is 2.2 minutes), and by having two levels of flagging. The upgrade in sophistication will also include pop-up reminder windows.
The G8 is strictly a clinical lab analyzer—there are no plans at this time for POC applications. Merschen says that going POC with its HPLC assay could result in the degradation of the technology. The time will come, she says, when HbA1c testing will replace the current standard glucose tolerance test, which, with its requirements for fasting and multiple measurements, is cumbersome compared to the HbA1c methodology. Currently invaluable for diabetes prognosis and the resulting prevention and/or management of diabetes complications, HbA1c testing some day may be the primary diagnostic tool in the detection of this widespread disease, Merschen says. She adds that the IFCC is currently attempting to globally standardize HbA1c, and if that happens, it would make it easier to use it diagnostically.
-
- More information on diabetes.
As the diabetes epidemic continues to spiral, there will be many opportunities for companies to strive for advances in detection and treatment, and they will surely outdo one another in technological achievements in both POC and clinical lab applications.
Although at this time there may be no end in sight to the rise in diabetes, that bleak prospect is what fuels the advances in technology.
Alan Ruskin is staff writer for CLP.
References
- Tang Z, Louie R, Payes M, Chang K, Kost G. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2(3): 349-362.
- Tang Z, Louie R, Lee J, Lee D, Miller E, Kost G. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062-1070.
- Tang Z, Du X, Louie R, Kost G: Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124: 577-582.
- Khan A, Vasquez Y, Gray J, Wians F, Kroll M. The variability of results between point-of-care testing glucose meters and the central laboratory analyzer. Arch Pathol Lab Med. 2006;130(10): 1527-1532.
- Van der Berghe G et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345:1359-1367.
- Lewis K, Kane-Gill S, Bobek M, Dasta J. Intensive insulin therapy in critically ill patients. Ann Pharmacother. 2004;38:1243-1251.
- Krinsley J. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004 Aug;79(8): 992-1000.
- Furnary AP et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thoracic Cardiovasc Surg. 2003 May;125(5): 1007-1012.






