An international team of researchers has developed a diagnostic platform that uses cell-free, synthetic biology tools and companion hardware to provide rapid, de-centralized, and low-cost patient testing for infectious diseases like the Zika virus.
This field-based patient trial for this diagnostic platform that uses cell-free, synthetic biology tools was conducted on-site in Latin America
Study results—published in Nature Biomedical Engineering and headed by experts from the University of Toronto’s Leslie Dan Faculty of Pharmacy—show that the synthetic biology platform has analytical specificity and sensitivity equivalent to a US Centre for Disease Control PCR test for Zika and a diagnostic accuracy of 98.5% with 268 patient samples collected in Recife, Brazil. The cell-free platform is also programmable and can be similarly applied to detect any pathogen sequence. In addition to validating highly accurate diagnostic results for Zika, the team also achieved similar diagnostic performance for chikungunya virus, another mosquito-borne arbovirus.
“We see emerging diagnostics, like the paper-based tests we’ve developed, as having tremendous near-term potential to augment existing PCR capacity, improve equity in access to health care, and aid in the responses to public health crises,” says Keith Pardee, assistant professor in the department of pharmaceutical sciences, Leslie Dan Faculty of Pharmacy, University of Toronto.
Zika Virus Outbreak
Prior to the current global COVID-19 pandemic, the 2015/2016 outbreak of Zika virus in Latin America emphasized the urgent need for rapid and low-cost testing that can be deployed beyond the reach of centralized diagnostic labs, explains Pardee who has a Canada Research Chair in Synthetic Biology and Human Health.
“We were investigating and developing this technology well before the COVID-19 pandemic brought these issues to light at the global level. We’ve now been able to apply it and validate it in a region of endemic disease, which is really promising because these tools are meant to enable health systems to better respond to future outbreaks of infectious disease, particularly in low-resource settings,” he says.
Portable Synthetic Biology Diagnostic Platform
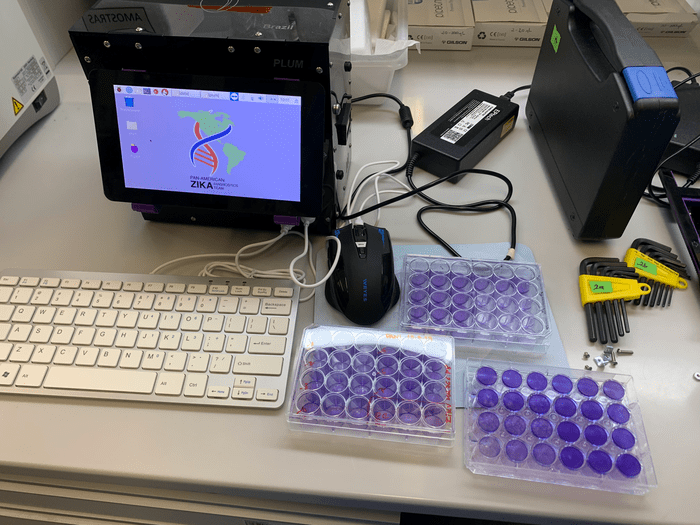
The portable, synthetic biology diagnostic platform is a combination of a cell-free, paper-based test and a field-ready companion device that allows data to be collected using image-based color analysis – purple for positive and yellow for negative. Called “PLUM” (portable, low-cost, user-friendly, multimode), the toaster-size reader presents results from up to 384 samples and displays them in a single image capture. The hardware and software that make up PLUM were originally developed by co-authors Livia Guo and Seray Çiçek as part of their graduate work in the Pardee lab. To keep production costs low, Guo and Çiçek, co-founders of LSK Technologies, used customizable software programs and off-the shelf electronics, enabling PLUM to be built for approximately $500 per unit.
On the molecular side, the cell-free, synthetic biology tests can be freeze-dried, allowing for distribution without refrigeration and, significantly, all of the molecular components of the test are independent of the PCR-supply chain.
“Here we have demonstrated that these two technologies combined create a low-cost, highly accurate diagnostic tool,” says study lead author Margot Karlikow, a postdoctoral fellow in the Pardee lab from 2016 to 2021 and now co-founder of En Carta Diagnostics. “We also demonstrated that it is feasible to transport the platform across a significant distance and implement it effectively in another country. In many low- and middle-income countries, there is no PCR testing available outside of main cities, so the ultimate goal is that this platform be used as a high-quality alternative to PCR in more regional settings.”
Lindomar Pena, PhD, department of virology, Oswaldo Cruz Foundation (Fiocruz), led the Brazilian team that collaborated on the project.
“This robust diagnostic platform displayed desirable features to be used in developing countries such as Brazil and in laboratories with basic infrastructure. We hope it can be further developed and deployed in the Brazilian network of public health laboratories to diagnose Zika patients, trace contacts and identify hot-spot areas with active community transmission,” he says.
Showing that the synthetic biology platform could be transported and accurately detect Zika virus in patient samples is a significant step forward in creating more accessible and de-centralized testing, says Pardee.
However, the extraction of RNA from patient samples still requires liquid handling by skilled technicians at this stage. “With performance on patient samples now validated, we are tackling these next challenges, like sample preparation, so that the platform and PCR-like diagnostic capacity can be distributed more broadly into the communities where they are needed.”
Featured image: A “lab-in-a-box”, the PLUM reader (Portable, Low-cost, User-friendly, Multimode), presents results from up to 384 patient samples and displays them in a single image capture. PLUM is also highly programmable and can be similarly applied to detect any pathogen sequence. Photo: Livia Guo, LSK Technologies