As women delay having children due to improved educational levels and participation in the workforce, rates of infertility have risen. Anti-mullerian hormone is commonly used as a marker to assess ovarian reserve and reproductive potential.
By Sara Love, PhD, DABCC, and Jeanne Rhea-McManus, PhD, MBA, DABCC, NRCC
The last five to six decades have seen a trend of postponing childbearing until later years, largely driven by increased female educational levels and participation in the labor force. This upward trend in the mean age at which women become pregnant has subsequently led to an increase in infertility1, which affects approximately 17% of couples worldwide who are trying to conceive2. For women under the age of 35 years, infertility is defined as the inability to become pregnant after at least one year of unprotected sex; for women over the age of 35, it is defined as the inability to become pregnant after just six months of unprotected sex. Infertility can be caused by a number of factors, with depletion of ovarian reserve being one of the most common causes.
What Is Ovarian Reserve?
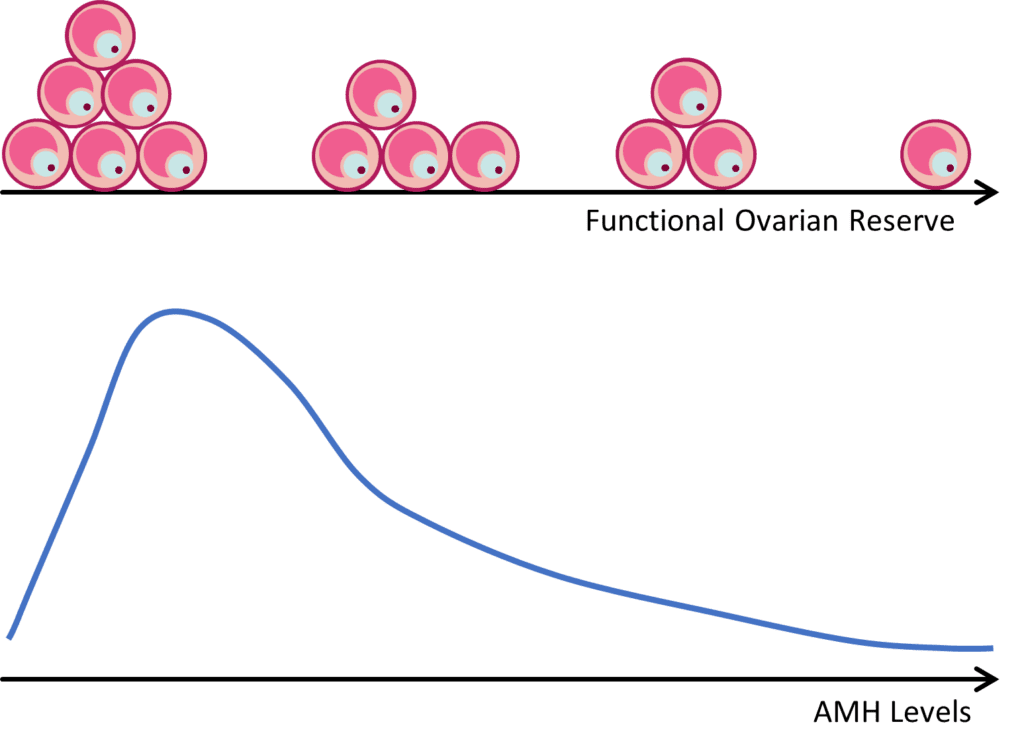
Ovarian reserve refers to the reproductive potential left within a woman’s ovaries and is based on the number and quality of eggs. Most females are born with around 1,000,000 eggs (in the form of primordial follicles)3. As a woman ages, eggs are continuously lost and no treatment or intervention can stop or prevent this natural decline. By the onset of puberty, most women have around 400,000 eggs left, about 27,000 eggs left by their late 30s, and around 1,000 eggs left at the onset of menopause. When there has been a loss of normal reproductive potential in the ovaries due to a lower number of remaining eggs, this is referred to as diminished ovarian reserve. While the most common cause of diminished ovarian reserve is aging, other causes include genetic defects and aggressive medical treatments, which may harm the reproductive system, such as radiation treatments for cancer.
How has Ovarian Reserve Traditionally Been Evaluated?
Primordial follicles remain in a dormant state until puberty. Due to their size and where they are located, it is not possible to directly assess the number of primordial follicles. However, as primordial follicles are recruited and develop into antral follicles, they become visible by ultrasound. Antral follicle count (AFC) continues to be the gold standard for assessing ovarian reserve and requires state-of-the-art technology operated by highly trained staff. The number of antral follicles is indicative of the number of primordial follicles remaining in the ovaries. In women with previous ovarian surgeries and/or cysts, AFC by transvaginal ultrasound is typically unsuitable as the conditions may obscure visualization of small follicles. Other limitations of AFC include its subjective nature and the variability that can be observed between cycles.
Follicle stimulating hormone (FSH) is responsible for the recruitment of antral follicles, one of which will ultimately emerge as the dominant follicle/mature egg. FSH has been the traditional blood-based biomarker for assessment of ovarian reserve and is typically measured on day three of the menstrual cycle. FSH levels vary throughout a woman’s cycle with a cycle day three measurement used as a baseline for how well a woman’s body may ultimately develop a mature egg. Lower FSH results are favorable and indicate normal ovarian reserve whereas high FSH levels suggest decreased egg supply. Limitations of FSH include the intra- and inter-cyclic fluctuations3 and its susceptibility to conditions other than ovarian causes such as hormonal therapy, oral contraception, and pituitary tumors.
The newest blood-based biomarker to gain attention for the assessment of ovarian reserve is anti-müllerian hormone (AMH).
What is Anti-Müllerian Hormone?
Identified in 1940s as the factor responsible for the regression of the Müllerian ducts in the male fetus, AMH is a homodimeric glycoprotein belonging to the transforming growth factor-beta superfamily. In the absence of AMH, the Müllerian ducts develop into the uterus, fallopian tubes, and upper part of the vagina. After roughly 50 years, AMH characterization ultimately demonstrated that AMH was also found in females. Similar to other hormones, the AMH gene encode a pre-pro form of AMH which is ultimately cleaved into the bioactive form; the physiological role of the various intermediate AMH forms and cleavage products are not completely understood. Unlike other reproductive hormones, AMH appears to be largely stable across the menstrual cycle although some evidence suggests there is between cycle variations intra-individually. In females, AMH expression has been observed as early as 32 weeks gestation in the ovaries.
AMH to Assess Ovarian Reserve
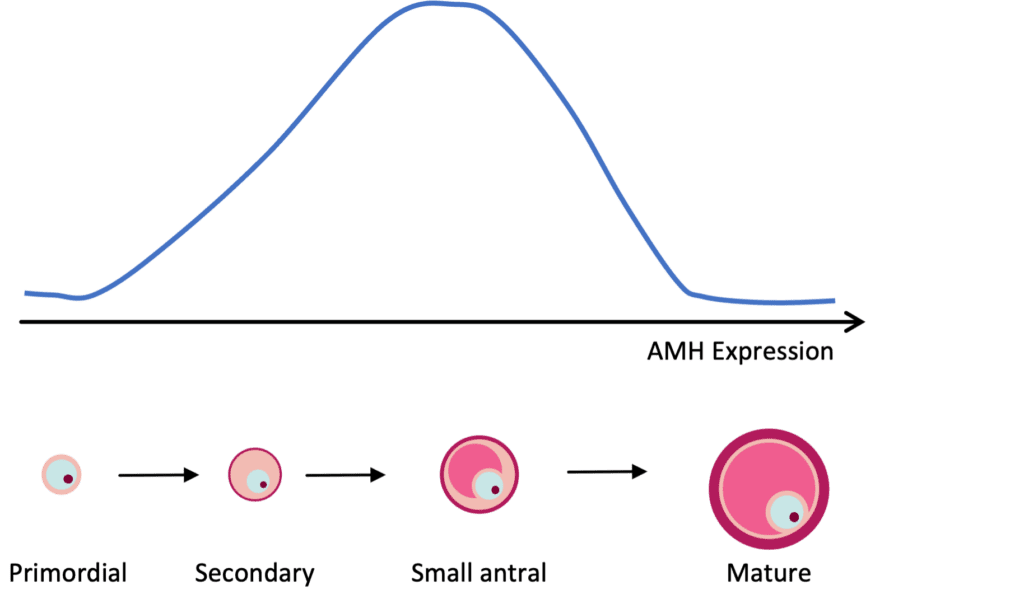
AMH expression can be detected in granulosa cells of follicles in the primary stage of development. AMH expression continues until the growing follicles reach the size (~8 mm) and differentiation state at which time one is selected for dominance. As mentioned previously, females are born with a finite number of primordial follicles and up to 1,000 can be recruited with each wave of activation, leading to the steady decline in the primordial follicle pool with age.
Because AMH is produced by growing follicles, a decrease in the primordial follicle pool will lead to a decrease in the number of growing follicles, and therefore a decrease in circulating AMH levels (Figure 1). A strong correlation between AMH and the number of antral follicles that are visible by ultrasound (AFC) has been well established. Thus, AMH is commonly used as a marker to assess ovarian reserve and reproductive potential.
Other AMH Uses
Prediction of Ovarian Response. Women suffering from infertility often undergo controlled ovarian stimulation (COS) to facilitate production of multiple oocytes as part of in vitro fertilization (IVF) protocols. One of the reported risks of COS is a life-threatening complication called ovarian hyperstimulation syndrome (OHHS), which is characterized by the degree of abdominal distention, ovarian enlargement and respiratory, hemodynamic, and metabolic complications4. The use of AMH measurements to assess a woman’s potential response to COS allows individualization of IVF stimulation protocols. By tailoring COS protocols to the patient, potential risk to the patient (e.g., development of OHSS) is minimized as are the need for costly investigations, hospitalizations and/or repeat outpatient visits due to OHSS.
AMH-tailored IVF protocols have also been found to reduce the incidence of failed fertilization leading to an unsuccessful cycle, thereby reducing the number of repeat cycles and further reducing cost of IVF5.
Prediction of Menopause. Menopause is defined as the permanent cessation of menstruation due to the loss of ovarian follicular activity and can occur any time after the age of 35. In the U.S., the average age of onset is 51 years. Menopause is a complex physiological process with no single test able to definitively predict its onset with a high degree of accuracy. Biomarkers such as inhibin B, FSH, and AFC have traditionally been used to predict the onset of menopause. However, because AMH regulates early follicular development, serum concentration decreases may be the earliest marker to predict menopause; thus, AMH has been described as the best endocrine marker to predict age-related decline in ovarian reserve by predicting median age to menopause. Recent studies have demonstrated that AMH may accurately reflect the transition of up to five years prior to menopause as women with very low AMH for their age are more likely to undergo premature menopause. AMH therefore may provide crucial information during family planning decisions, especially for women who wish to postpone pregnancy.
AMH and diagnosis of polycystic ovarian syndrome (PCOS). PCOS is an endocrine disease affecting approximately 5 million women in the U.S. The most common symptoms of PCOS include high levels of androgen hormones, missed or irregular periods, acne, hirsutism, and a high number of small cysts on the ovaries. One of the criteria that can be used for diagnosis of PCOS is the presence of polycystic ovaries, defined as ≥12 follicles from 2 to 9 mm per ovary. The threshold of ≥12 follicles remains at the center of debate as the use of this cutoff may lead to an over-diagnosis of PCOS. In addition, the subjective nature of counting follicles by ultrasound results in a lack of standardization that can also lead to differences in diagnosis. Because the ovaries of women with PCOS have an increased number of AMH-secreting preantral and small antral follicles leading the AMH elevations up to two- to threefold, AMH has been described as an attractive candidate biomarker of PCOS6.
Potential role for AMH in oncology. A variety of potential future uses have been suggested in the literature as possible roles for AMH in onco-fertility, particularly in younger women, though these applications are at the early stages. Cancer applications provide a variety of opportunities for AMH including as a potential tumor marker, such as in granulosa cell tumors where AMH has found to be elevated. Another role for AMH is in cancer related fertility preservation where AMH ovarian reserve assessments can guide decision making for preservation options like elective cryopreservation when treatment will include chemo- or radiotherapies.
While more data are needed to support the utility of AMH in the prediction of menopause, diagnosis of PCOS, and decision making in cancer related fertility preservation, it is a widely accepted as a tool that can provide helpful information regarding a woman’s fertility and/or reproductive health.
ABOUT THE AUTHORS

Sara Love, PhD, DABCC, is a board-certified clinical chemist and medical officer for Siemens Healthineers.

Jeanne Rhea-McManus, PhD, MBA, DABCC, NRCC, has been with Siemens Healthineers for 8 years, previously as a Medical Officer and currently as the Sr Director of Medical Science Information and Communication.
References
- Sadeghi MR. Realities and Hopes in Social Freezing: A Developing Practice to Stop Reproductive Ageing. J Reprod Infertil. Jan-Mar 2023;24(1):1-2. doi:10.18502/jri.v24i1.11902
- WHO. 1 in 6 people globally affected by infertility: WHO. Accessed 4/14/2023, https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility
- Practice Committee of the American Society for Reproductive Medicine. Electronic address aao, Practice Committee of the American Society for Reproductive M. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. Dec 2020;114(6):1151-1157. doi:10.1016/j.fertnstert.2020.09.134
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod. Sep 1 2017;32(9):1786-1801. doi:10.1093/humrep/dex234
- Yates AP, Rustamov O, Roberts SA, et al. Anti-Mullerian hormone-tailored stimulation protocols improve outcomes whilst reducing adverse effects and costs of IVF. Hum Reprod. Sep 2011;26(9):2353-62. doi:10.1093/humrep/der182
- Jamil Z, Fatima SS, Ahmed K, Malik R. Anti-Mullerian Hormone: Above and Beyond Conventional Ovarian Reserve Markers. Dis Markers. 2016;2016:5246217. doi:10.1155/2016/5246217
- Moolhuijsen LME, Visser JA. Anti-Mullerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J Clin Endocrinol Metab. Nov 1 2020;105(11):3361-73. doi:10.1210/clinem/dgaa513




