Laboratory workflow is unique within health care. It offers a clinical product in which extremely high volumes must be delivered with a high degree of accuracy. But in addition to its central role in health care diagnosis and treatment, laboratory information is a critical element in achieving the communication, interoperability, and integration goals embodied in the health care reform process. The lab plays a vital role in meeting reportable results meaningful use (MU) goals and ensuring the connectivity to community-based providers that is key to the success of accountable care organizations (ACOs). It also should play the lead role in housing and maintaining Logical Observation Identifiers Names and Codes (LOINC®).
LABORATORY IS AT THE HEART OF HEALTH CARE DECISIONS
Laboratory results are a critical part of the health care process. While laboratory tests make up only about 2.3 cents of every dollar spent on health care, laboratory tests result data account for approximately 70% of patients’ medical records and affect between 70% and 80% of the clinical decisions made. Sophisticated use of information technology enhances the value of this lab test data.
Hospital laboratory workflow is also unlike that of any other area of the hospital. Laboratory records associated with managing a specimen once it has been obtained are very different from records developed to document patient care, derive a diagnosis, monitor the treatment plan, or administer medication.
The combination of such a high volume and the diagnostic value of the information means that hospitals can ill afford any kind of hiccups in their system. It also means that it is absolutely critical that the LIS have the capability to communicate accurate, reliable laboratory results to care providers who make decisions.
This very communication is at the heart of initiatives to reduce medical errors and reduce health care costs, and key to receiving incentives for meeting meaningful use (MU) objectives under the Health Information Technology for Economic and Clinical Health Act (HITECH Act). HITECH was enacted as part of the American Recovery and Reinvestment Act of 2009 to foster the adoption and meaningful use of health information technology.
Interoperability among data systems is a key theme throughout the meaningful use definition.
The meaningful use criteria requires electronically capturing health information in a standard format, using that information to track key clinical conditions, communicating that information for care coordination purposes, and initiating the reporting of clinical quality measures and public health information.
Reporting of public health information is a laboratory’s core competency. For example, Sunquest Inc, Tucson, Ariz, has implemented systems for more than 17 public health agencies, and Sunquest Laboratory was the first LIS to be modularly certified for the Reportable Results criteria.
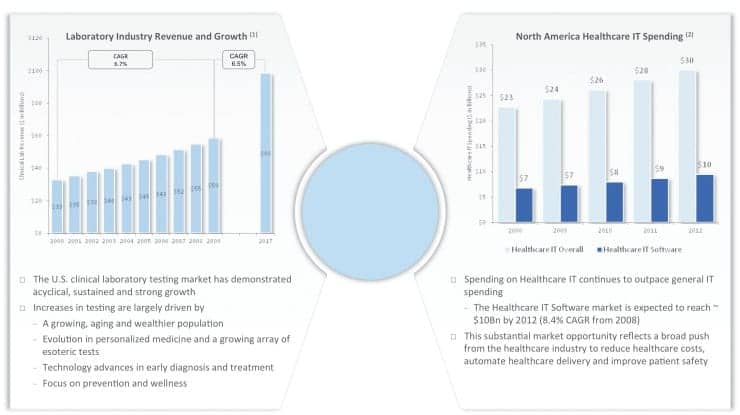
Figure 1
Physician portals are another way to meet MU criteria, including computerized physician order entry (CPOE). The laboratory is in an excellent position to help physicians meet the notoriously difficult CPOE criterion by providing a modularly certified portal for entering laboratory orders. This offers a simple way for physicians to demonstrate a difficult MU criterion at little or no cost to them, while at the same time helping hospitals and reference labs increase physician affinity and grow their outreach business.
A physician portal into the lab can also provide real-time results to the physician. This is important because the ultimate vision of MU is one in which providers have real-time access to all medical information and tools to help ensure the quality and safety of the care provided, all while affording improved access and elimination of health care disparities.
LAB VOLUME/UTILIZATION CENTRAL TO HEALTH CARE FUTURE
As shown in Figure 1, laboratory test volume and utilization has been growing, driven largely by demographic trends as the Baby Boom generation ages. CMS data suggests that prior to age 65, patients utilize an average of two laboratory tests per year, while after age 65, they utilize about nine tests per year. Other trends driving the increase include the evolution in personalized medicine and the growing array of esoteric tests, technology advances in early diagnosis and treatment, and an increased focus on prevention and wellness.
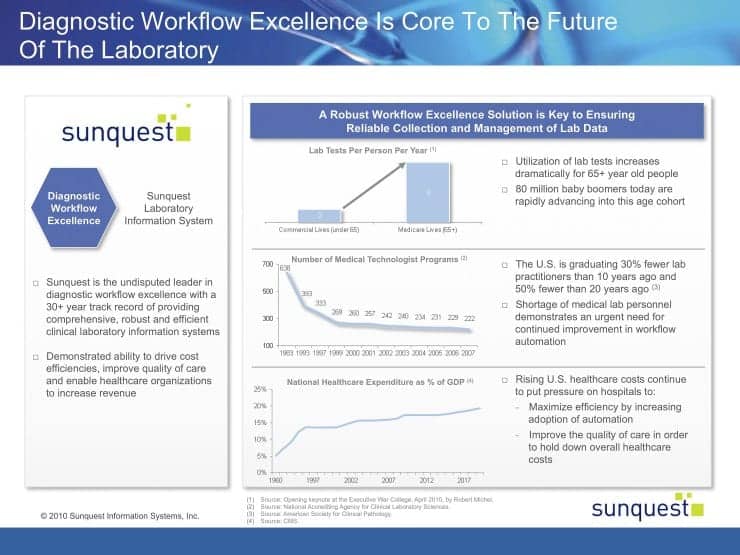
Figure 2
In recent years, as test volumes have increased due to demographic and other trends, medical technician and related programs actually decreased, creating the demand for technology to help hospitals improve handling of large volumes with fewer staff members. Figure 2 illustrates these trends graphically. The mismatch between the growth in tests done and the number of technicians available to conduct laboratory analyses has resulted in a situation in which the huge volume increases have required automated robotic lines and LIS solutions such as specimen routing and tracking to help technicians work through the volume with minimal variation and a high rate of predictability.
HEALTH CARE REFORM—FROM MEANINGFUL USE TO ACCOUNTABLE CARE ORGANIZATIONS
MU, established under the 2009 HITECH Act, offers significant financial incentives for investment in information technologies. These in turn drive interoperability and integration to promote clinical decision support leading to changes in the delivery of care.
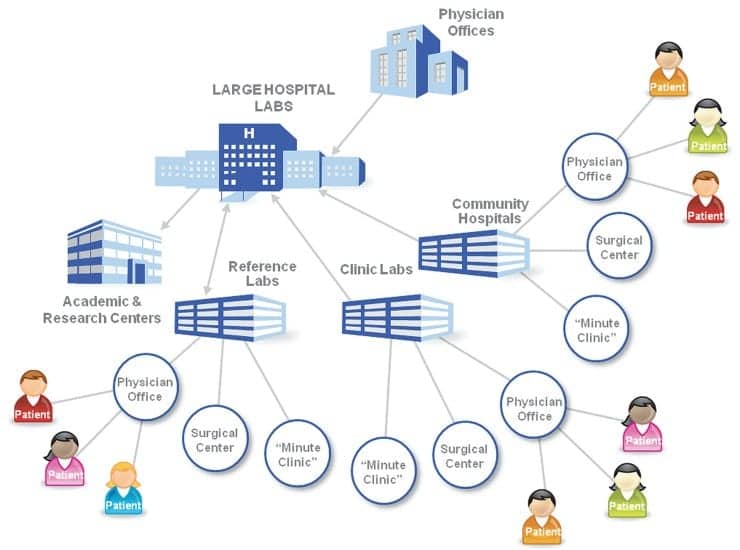
Figure 3
In 2011, the health care reform process resulted in the Affordable Care Act, establishing the accountable care organization (ACO), which encourages the development of integrated care delivery networks accountable for the care of a population of patients. In the ACO model, all patients are fully engaged in their health care.
There has been a logical progression from HITECH’s MU to the ACO, and the laboratory must be engaged throughout the transition. First, the lab must establish the technology and interoperability, and then it can engage the patients and change the paradigm to put patients at the center of the model.
This progression is shown graphically in Figures 3 and 4. Figure 3 shows the importance of the laboratory to the MU criteria. Electronic capture is the first step toward the ability to pass information between entities. The hospital is critical to the MU objectives, as it is strategically the center of patient care coordination with a network of associated labs and physicians. Laboratory data is also one of the most highly requested pieces of information throughout the patient care continuum.
In Figure 4, we see that in the ACO schema, the players are the same and the hospital is still a strategic asset, with the laboratory still producing the important data, but the patient is at the center.
ACO actually presents an opportunity for the laboratory to add value. The lab is already an important and primary integrator of clinical information within the care continuum. In the ACO model, more care will be delivered in ambulatory settings, making outreach solutions essential. In addition, connectivity to community-based providers is key to the success of the ACO.
MEANINGFUL USE, ACCOUNTABLE CARE ORGANIZATIONS, AND LOINC
One additional critical feature of both MU and the interoperability needed to enable ACOs is LOINC, a universal code system for identifying laboratory and clinical observations.
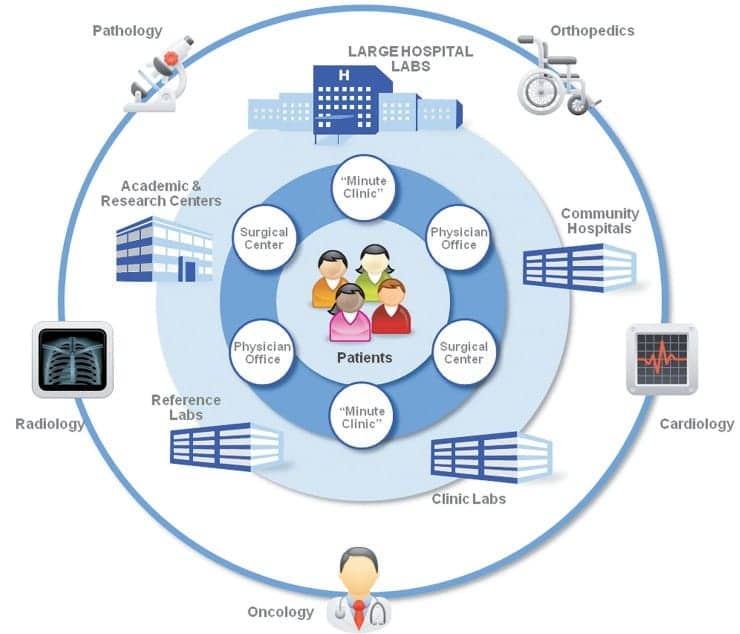
Figure 4
LOINC was developed in 1994 by The Regenstrief Institute Inc, which is supported by the Regenstrief Foundation and closely affiliated with the Indiana University School of Medicine and the Health and Hospital Corp of Marion County, Ind. LOINC provides a definitive standard for identifying clinical information in electronic reports.
The LOINC database provides a set of universal names and ID codes for identifying laboratory and clinical test results in the context of existing HL7, ASTM E1238, and CEN TC251 observation report messages. LOINC codes are intended to identify the test result or clinical observation. Other fields in the message can transmit the identity of the source laboratory and special details about the sample.1 It is important to note that a care system cannot fully “understand” and properly file the results they receive unless they either adopt the producer’s laboratory codes (which is impossible if they receive results from multiple sources), or invest in the work of standardizing to LOINC.
LIS, Middleware Resources
- Antek HealthWare, a CompuGroup Medical Company
(800) 359-0911; www.antekhealthware.com - Data Unlimited International Inc
(240) 631-7933; www.duii.com - Dawning Technologies Inc
(800) 332-0499; www.dawning.com - HEX Laboratory Systems
(800) 729-2085; www.hexlab.com - Orchard Software
(800) 856-1948; www.orchardsoft.com - Psyche Systems Corp
(760) 603-7200; www.psychesystems.com - SCC Soft Computer
(727) 789-0100; www.softcomputer.com - Siemens Healthcare Diagnostics Inc
(888) 588-3916; www.siemens.com/diagnostics - Sunquest Information Systems
(877) 239-6337; www.sunquestinfo.com - Sysmex America Inc
(847) 996-4500; www.sysmex.com/us - Thermo Fisher Scientific Inc
(866) 463-6522; www.thermofisher.com
LOINC is absolutely essential for interoperability, as it facilitates the exchange and pooling of results for clinical care, outcomes management, and research. It will be an essential part of standardization necessary for both MU and ACOs, since LOINC codes are universal identifiers for the laboratory, replacing “local” internal, idiosyncratic code values.
Since LOINC is mainly a laboratory “vocabulary” and will be used in the lab and in interfaces from the lab, LOINC should be stored and maintained in the laboratory by lab personnel. The test dictionary has to be maintained in the laboratory system—one would not want to duplicate it and have to maintain it in both the LIS and the HIS. It is important that the laboratory system be capable of sending the LOINC-coded data where it is needed.
THE ROLE OF MIDDLEWARE
Middleware has been defined as the software layer that lies between the operating system and the applications on each site of the system.2
Middleware is widely being used to translate legacy standards and proprietary vocabularies into the data exchange standards now required. Take the example of auto filing or auto verification. When clinical validation of normal results is not required, auto verification can be used. That means setting rules and maintenance so the system can automatically file results and send the reports on to the receiving systems/physicians without human intervention to review the results. Many laboratories auto file “normal” results and only require technicians to review abnormal results or results that fail quality control procedures. This can make a significant impact on efficiency and turnaround time.
We have found that Sunquest clients are routinely auto-verifying more than 85% of their core laboratory results, freeing up staff for other lab-related tasks. Since a key goal of MU is to reduce the cost of health care in America, and ACOs will be compensated by way of shared savings, auto filing is a great way to achieve savings.
However, LIS systems that rely on middleware for instrument interfacing and auto verification of results tend to increase overhead. Even if a robust middleware has easy-to-understand rules and maintenance, and a variety of choices to stop auto file when appropriate, technicians will need to monitor the LIS resulting and the middleware. To avoid redundancy, technicians should only have to monitor the end point rather than also monitor the middleware.
Some hospitals are considering data exchange middleware to handle LOINC mapping. However, this requires either a translation table or for the translation to take place on the interface engine or middleware. To accomplish this, the middleware needs the local laboratory code information. Users will not be able to maintain the laboratory code solely in middleware because these codes are core to the LIS, so to have it in middleware LOINC would have to be maintained in two places. Most laboratories are wary of maintaining the lab dictionary in two places, whether the second place is the HIS or middleware, because it is very difficult to keep them in synch.
Therefore, for both auto verification and LOINC, middleware creates redundancies that increase overhead.
FINAL THOUGHTS
The laboratory plays a critical role in delivering a clinical product and is at the center of the evolving care delivery models now unfolding.
The paradigms, including MU, ACO, and LOINC, all require more interoperability and standardized nomenclature. The laboratory is the source of LOINC, and health care systems need robust and flexible solutions to help them map and manage LOINC and meet MU and ACO requirements.
REFERENCES
- LOINC Overview. Available at: loinc.org/faq/getting-started/getting-started/#what-is-loinc Accessed November 29, 2011.
- ObjectWeb. What is Middleware? Available at: middleware.objectweb.org. Accessed November 29, 2011.





