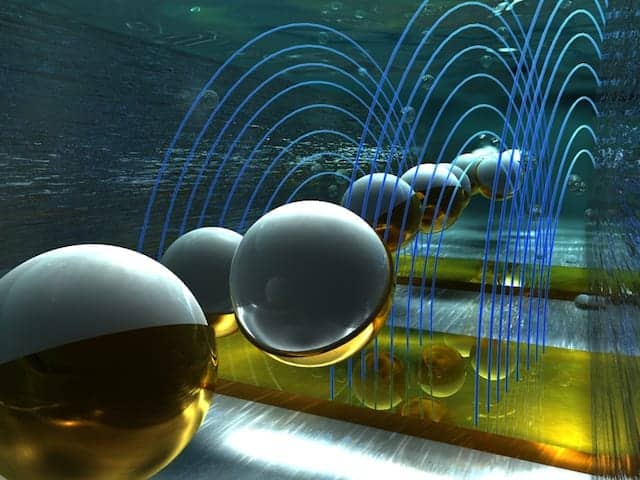
Barcoded microparticles promise ultracompact detection of pathogens and pollutants
Interview by Steve Halasey
Optical instruments are the state-of-the-art technology for detecting and measuring biomarkers, but such devices are typically too large to be considered portable.
Addressing this issue, engineers at Rutgers University have recently invented a biosensor technology that could be used in handheld or wearable devices to monitor an individual’s health and exposure to dangerous bacteria, viruses, and pollutants.1
The technology involves electronically barcoding microparticles, which can then be used to simultaneously test for multiple health and disease indicators, including bacteria and viruses as well as airborne and other contaminants. A study describing the invention was recently highlighted on the cover of Lab on a Chip, a journal published by the Royal Society of Chemistry.2
Electronic detection of microparticles allows for the development of ultracompact instruments small enough for use in wearable devices. The Rutgers researchers’ technique for barcoding particles is, for the first time, fully electronic, enabling biosensors to be reduced to the size of a wearable band or microchip.
“This is really important in the context of personalized medicine or personalized health monitoring,” says Mehdi Javanmard, PhD, an assistant professor of electrical and computer engineering at Rutgers University, New Brunswick, and senior author of the study. “Our technology enables true labs on chips. We’re talking about platforms the size of a USB flash drive, or something that can be integrated into an Apple Watch, for example, or a Fitbit.”
The multiplexing capability of the Rutgers biosensor technology is also an important feature. In recent decades, research on biomarkers—indicators of health and disease such as proteins or nucleic acid molecules—has revealed the complex nature of the molecular mechanisms behind human disease. That has heightened the importance of testing body fluids for numerous biomarkers simultaneously, the study says.
“One biomarker is often insufficient to pinpoint a specific disease because of the heterogeneous nature of various types of diseases, such as heart disease, cancer, and inflammatory disease,” says Javanmard. “To get an accurate diagnosis and accurate management of various health conditions, you need to be able to analyze multiple biomarkers at the same time.”
According to Javanmard, the technology is greater than 95% accurate in identifying biomarkers, and fine-tuning is under way to make it 100% accurate.
To find out more about the potential of this recent advance for diagnostic applications, CLP recently spoke with Mehdi Javanmard, PhD, an assistant professor of electrical and computer engineering at Rutgers University, New Brunswick, and senior author of the Lab on a Chip study.
CLP: In the diagnostics sector, miniaturization has been going on for what seems like forever. Is there still an unmet need for smaller diagnostic devices?
Mehdi Javanmard, PhD: Yes, definitely so, and this will continue to be the case for the foreseeable future. In the context of health monitoring, environmental monitoring, food monitoring, and agriculture, there are needs for smaller and smaller low-powered sensors that can continuously monitor panels of relevant molecules for diagnostic purposes.
In the specific context of health monitoring, we lack tools that can continuously quantify key biomarkers over time, despite the fact that such tools could be very useful for both chronic and acute diseases. Consider the examples of asthma and other respiratory inflammatory conditions. Devices that can monitor inflammation over time could be immensely useful in predicting exacerbations. Many hospitalizations would be prevented if asthmatics could be notified in advance that they’re about to have an asthma attack, and that they need to take their inhaler.
CLP: What previously unmet needs does your new technology address?
Javanmard: When compared to optical methods of biomarker detection, electrical detection of biomarkers generally allows for more-miniaturized platforms because of the method’s ability to piggyback onto integrated circuit technologies that have been refined by the computer chip–making industry over the past several decades. However, with electrical detection, it is difficult to detect multiple types of biomarkers simultaneously. Our method of electronic barcoding of particles eliminates this key bottleneck.
CLP: What were the previous obstacles or hurdles that needed to be overcome? Were these primarily conceptual, technical, or something else?
Javanmard: Figuring out a way to electronically barcode particles has been both a key conceptual and technical challenge. In part, this is because past methods used to measure the impedance of a particle have been mostly limited to obtaining information about the volume of the particle.
By combining bottom-up synthesis techniques (self-assembly) with top-down fabrication techniques (thin-film deposition), we were able to fabricate nanocapacitors on the sides of the particles, giving each particle a unique signature, or barcode. The problem was solved by using a multidisciplinary approach, borrowing solutions from biology, chemistry, electronics, and materials science.

Artist’s rendition of barcoded microparticles flowing through a channel and passing through electric fields, where they are detected electronically and barcode-scanned. Image by Ella Marushchenko and Alexander Tokarev/Ella Maru Studios courtesy Rutgers University.
CLP: How confident are you that your technology can be employed to create portable diagnostic monitors suitable for patient care?
Javanmard: So far, our data look very promising. We’re optimistic about the technology.
CLP: What are the advantages of a highly portable, or even wearable, approach to patient monitoring?
Javanmard: With benchtop instruments, samples need to be collected in large volumes, stored, shipped, and transferred to the lab, where analysis is performed. Finally, after some time, results are made available to the physician and the patient. With highly portable or wearable biomarker monitoring devices, that entire process can be bypassed. Portability enables diagnostic tools to be brought directly to the patient, providing real-time results and nearly instantaneous feedback on which the medical professional or patient can act.
CLP: As your study suggests, clinical laboratorians are familiar with the bead-based system by Luminex as a predecessor of your technology. In what ways is your approach different?
Javanmard: The Luminex system is a very powerful technology for optically barcoding beads with very high density multiplexing, and is great for benchtop biomarker analysis. Our technology is electronic, and thus allows for making an ultracompact instrument.
CLP: Defining distinct barcoded microparticles and refining the processes to produce them both seem like very complex tasks. What new insights did you have about these tasks? Do you think they can be automated?
Javanmard: Certainly. The techniques we’re using—self-assembly and thin-film deposition—are compatible with high-volume manufacturing, so automation would be straightforward. Also, we’re not barcoding one particle at a time. Particles would be barcoded in batch format. That’s something the manufacturer would do once. Users wouldn’t have to worry about doing the barcoding, as the barcoded particles would be ready to use in their assays.
CLP: Your Lab on a Chip study defines some limitations that apply to electronically barcoded microparticles, including a somewhat low number of definable barcodes—on the order of 20 to 30. How does this compete with other approaches that can process “barcoding densities on the order of thousands,” as your study says?
Javanmard: High-density barcoding on the order of thousands is desirable for benchtop applications, such as those used for biomarker discovery. For applications where portability is important, like health monitoring, the user would be more interested in monitoring a handful of validated biomarkers.
CLP: Would it be possible to reassign a barcode for use in syndrome-distinct multiplexed test cartridges—one for respiratory diseases, another for gastrointestinal, a third for cardiology?
Javanmard: Yes, exactly! Kits geared toward different applications can be developed, and this is a key advantage of using barcoded particles. The particles themselves are agnostic to the biomolecule being targeted. So long as an appropriate biorecognition element is functionalized on the surface of the particle, the sky is the limit as to what can be detected.
CLP: How do you see this technology progressing toward commercialization? For instance, are you considering spinning the intellectual property out to be a separate company, or do you see your exit as sale to or merger with another company?
Javanmard: All options are on the table!
Steve Halasey is chief editor of CLP.
References
- Bates TB. Lab on a chip could monitor health, germs, and pollutants [press release]. New Brunswick, NJ: Rutgers University, 2017. Available at: http://news.rutgers.edu/research-news/lab-chip-could-monitor-health-germs-and-pollutants/20170612#.wugezhpytca. Accessed June 14, 2017.
- Xie P, Cao X, Lin Z, Javanmard M. Top-down fabrication meets bottom-up synthesis for nanoelectronic barcoding of microparticles. Lab Chip. 2017;17(11):1939–1947; doi: 10.1039/c7lc00035a.