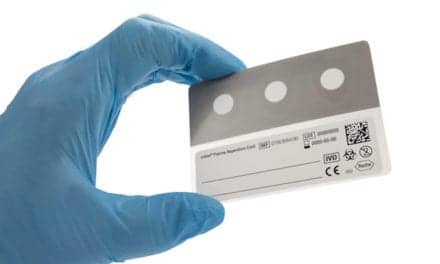
In the wake of a nuclear disaster, the REDI-Dx can help responders triage patients by rapidly providing data about their levels of absorbed ionizing radiation. The test uses a blood sample collected by fingerstick or venipuncture to measure the relative expression of a panel of radiation-sensitive genes. According to Ilja Bonsen, founder and managing director of IB Consultancy, an independent defense and security firm specializing in non-conventional threats, REDI-Dx was selected because “it has a clear end-user benefit, economic efficiency and an excellent match between requirements and the offered solution.”
REDI-Dx was developed as part of an ongoing collaboration between DxTerity Diagnostics and Duke University Medical Center, with funding from the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA).
For more information, visit DxTerity.