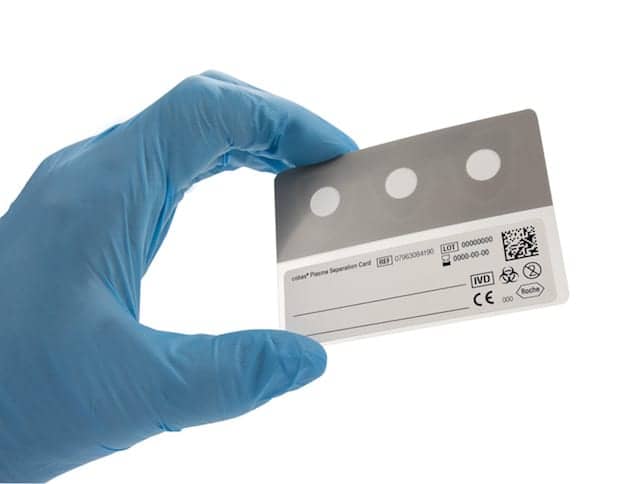
Roche Diagnostics, Basel, Switzerland, has launched the Cobas plasma separation card, a sample collection device for human immunodeficiency virus (HIV) plasma viral load testing. By collecting a small amount of a patient’s blood from a fingertip, the card is designed to simplify blood collection and sample transportation.
Traditionally, plasma viral load results required blood samples to be cooled during transport to the lab. The new Cobas plasma separation card fundamentally changes the way plasma samples are taken and processed. The card allows for reliable quantitative testing of patients with HIV living in remote areas—even areas of extreme heat and humidity—while meeting requirements of the World Health Organization (WHO) for determining HIV viral load prior to setting treatment.
“With the launch of the Cobas plasma separation card, we strengthen Roche’s ongoing commitment to providing life-saving diagnostics in the fight against HIV and AIDS,” says Roland Diggelmann, chief executive of Roche Diagnostics. “This card will improve access and increase HIV diagnostics, scaling up efforts to further the critical work of our many healthcare partners in eradicating the HIV/AIDS epidemic.”
The card will be added to the Roche global access program as a solution to help expand access to diagnostics in countries hardest hit by HIV. In partnership with the Clinton Health Access Initiative, the Global Fund, the Joint United Nations Program on HIV/AIDS, Unitaid, and the US Emergency Plan for AIDS Relief, Roche has been working to increase access to HIV diagnostic solutions to achieve the UNAIDS 90-90-90 goal to improve people knowing their status, getting on HIV therapy, and suppressing the virus from replicating.1
The card is being launched in countries accepting CE-mark certification, for use with the Cobas AmpliPrep/Cobas TaqMan HIV-1, and Cobas 6800/8800 HIV-1 tests.
The card meets the WHO sensitivity standard of <1000 cp/mL. It is stable under high heat and humid conditions, and provides results that correlate well to traditional plasma-based testing, which is considered the gold standard sample type for monitoring patients’ response to treatment.
For more information, visit Roche.
REFERENCE
- 90-90-90 Treatment for All [online]. Geneva, Switzerland: UNAIDS, 2018. Available at: www.unaids.org/en/resources/909090. Accessed February 5, 2018.