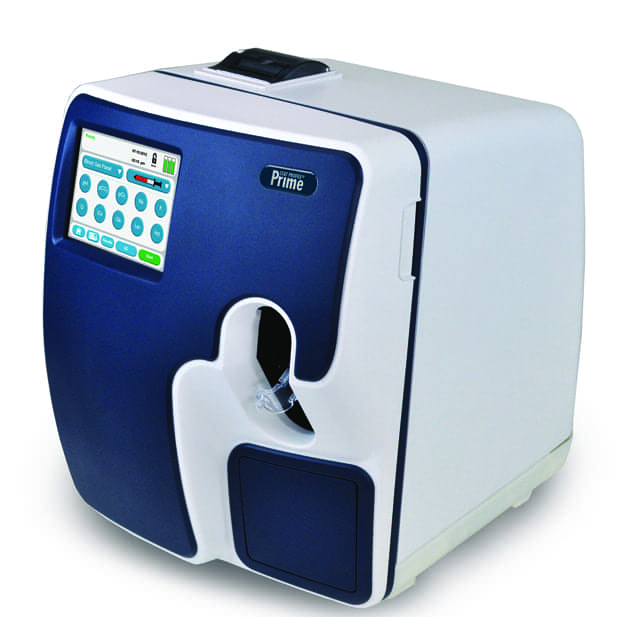
The Stat Profile Prime blood gas analyzer from Nova Biomedical, Waltham, Mass, has received FDA clearance for point-of-care (POC) use, enabling non-laboratory personnel to perform bedside, critical care testing.
The POC study submitted to FDA to achieve this clearance included testing performed by 53 non-laboratory professionals in three POC settings. Method comparison and precision studies demonstrated that the analyzer provides lab-quality results and is simple enough to use in POC settings, whether laboratory or POC personnel perform the testing.
The analyzer’s zero maintenance cartridge technology consists of individual cartridges for sensors and calibrators. Each cartridge is maintenance free, ready to use, and replaceable in seconds. As a result, cartridges carry an optimized lifespan, and analyzer uptime is improved. The design also eliminates waste, downtime, and higher costs of older, combined sensor and calibrator cartridges.
The company’s microsensor card technology contains biosensors for Cl, Glu, Hct, iCa, K, Lac, Na, PCO2, pH, and PO2. The card is automatically calibrated and ready to deliver a full 10-test profile in 60 seconds. The analyzer’s clot block sample flow path protects the card from blockages and prolonged downtime caused by blood clots.
Compact, lightweight, and featuring a color touchscreen, Stat Profile Prime may be used in a fixed location virtually anywhere in the hospital, or operated on a mobile cart with a battery back-up.
The analyzer will be showcased at AACC booth 1226.
For more information, visit Nova Biomedical.