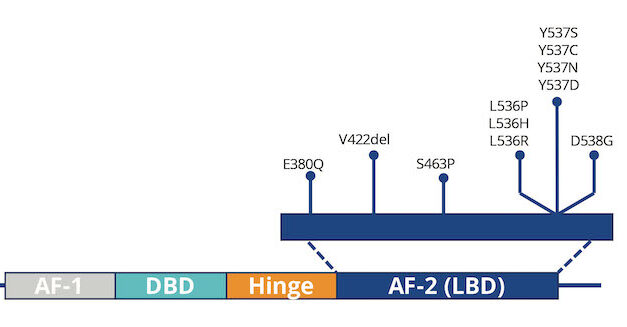
Researchers Identify Four New Markers for Detecting Aggressive Breast Cancer Cells in Blood
Researchers at Baylor College of Medicine have identified four new protein markers that improve detection of triple-negative breast cancer cells in blood, potentially enhancing the accuracy of liquid biopsy tests, according to a recent study.