Clinical Lab Products, in partnership with the research department at Mizuho Securities USA, recently surveyed a subset of CLP ’s audience to gauge expectations of clinical lab trends over the next 12 months, with an emphasis on personalized medicine. A summary of the survey results follows.
Real-time PCR and digital PCR rank highest as the most exciting technologies. Respondents expect significant implementation of genetic sequencing in diagnostic labs in 5 to 10 years, and surprisingly, implementation of mass spectrometry could be even sooner.
Users say two of the major diagnostics system providers, Siemens and Abbott Laboratories, would benefit most from increases in lab spending; Bio-Rad and Becton, Dickinson and Company also ranked well on the strength of microbiology and molecular diagnostic franchises. Beyond the big box manufacturers in the clinical diagnostic lab, standout companies included Becton, Dickinson; Cepheid; Bio-Rad; and Thermo Fisher.
For personalized medicine products, Siemens, Cepheid, Illumina, LabCorp, Quest Diagnostics, and Roche were ranked well. We were surprised by the recognition Illumina and Cepheid garnered considering the target audiences focus on large-scale classical or clinical diagnostics.
You told us personalized medicine is still in the very early stages of adoption in clinical reference laboratories—only 12% of respondents said they currently use personalized medicine tests, and only 18% said they were likely to add them, which suggests that molecular diagnostics has a long-term sustainable growth driver as these tests are adopted by the significantly larger clinical market.
Two tests stood out as potential adds over the next 12 months: HPV and infectious disease testing.
Respondents said that oncology—as expected—should see the greatest benefit from personalized medicine, and HER2 was the most-used personalized diagnostic test. Autoimmune/allergy ranked well for benefit from personalized medicine testing.
The adoption of personalized medicine has been hindered by the cost and insurance coverage. You said the greatest benefit, by far, is that it provides more targeted treatment.
Myriad’s technical leadership was displayed, over and above patent issues, with respondents overwhelmingly saying they would not offer a BRCA test even if patents were not an issue, due to their inability to match Myriad’s technical capabilities and knowledge of BRCA variations/clinical outcomes.
Demographics — US Labs, Directors and Technologists
Approximately 63% of respondents were lab directors or managers, and approximately 28% were technologists (see Exhibit 1). All respondents were US-based.
Exhibit 1. Demographics — US-based lab directors, technologists

[Source: CLP and Mizuho Securities USA Survey]
Survey method: An invitation to the 15-question survey was sent by e-mail to a subset of CLP ’s audience, and responses were collected online. Sixty-one percent of respondents completed each question. Each exhibit details the total number of respondents. The survey was conducted from June 9 to 15, 2011.
Based on a weighted average of responses, lab volume for respondents was approximately 1,325 requisitions per day (see Exhibit 2). The majority of respondents (more than 70) had volumes between 50 and 4,000 requisitions per day.
Exhibit 2.
What is the approximate daily volume of your lab, requisitions per day?

[Source: CLP and Mizuho Securities USA Survey]
Real-Time and Digital PCR Rank Highest Among Diagnostic Technologies
Real-time and digital PCR were ranked as the most exciting technologies in the clinical lab (see Exhibit 3). We attribute the solid support for digital PCR and real-time PCR to the fact that PCR is the backbone of molecular diagnostic testing.
With point-of-care testing (POCT), despite garnering the second-most votes for “exciting technology” after PCR technologies, opinions were mixed and POCT also earned second place for “least exciting” technology. We attribute the mixed responses to the potentially disruptive effect POCT technology could have on disease management and delivery. On a net-vote basis, next-generation sequencing (NGS) appears “more exciting” than “traditional” capillary sequencing (CE). While CE has made greater strides than NGS into the conventional diagnostic setting, NGS has a greater potential impact with the ability to offer whole-genome analysis at a fraction of the cost and price.
Microfluidics was the “least-exciting” technology, and mass spectrometry also had unfavorable scores for clinical technologies. Routine mass spec use in the clinic is a long-term development, and could remain a niche part of diagnostic testing.
Significant Sequencing Implementation is Expected in 5 to 10 Years. Surprisingly, Mass Spec Looks Sooner
Approximately 74% of respondents expect DNA sequencing to substantially replace existing diagnostic technologies in the next two decades, with 38% expecting this to occur in 5 to 10 years (see Exhibit 4).
Approximately 61% of respondents expect mass spectrometry to substantially replace existing diagnostic technologies in the next 2 decades. Twenty-five percent of those who responded expect it in 5 to 10 years—less than that for DNA sequencing, which fits with the lack of enthusiasm around clinical mass spectrometry in Exhibit 3.
Exhibit 3. What will be the most and least exciting product developments affecting the diagnostic industry during the next year?

Negative numbers indicate “least exciting” responses, or a net number of respondents believing the technology was “least exciting.”
[Source: CLP and Mizuho Securities USA Survey]
Taking the midpoint of ranges and weighted by response, mass spec is expected to significantly replace diagnostic technologies in approximately 7.5 years, and 8.5 years for DNA sequencing (range of 5 to 11 years)—surprisingly, in light of the lack of excitement for mass spec in Exhibit 3.
Exhibit 4: When will DNA sequencing and mass spectrometry substantially replace existing diagnostic technologies?

[Source: CLP and Mizuho Securities USA Survey]
Beyond the Major Clinical Diagnostic Vendors; Becton, Cepheid, Bio-Rad, and Thermo Rank Well for In-Demand, Quality, and Personalized Medicine
Exhibit 5. Which vendors will benefit from increased spending, have the best-in-class products, and are best for personalized medicine?

[Source: CLP and Mizuho Securities USA Survey]
Among the major box providers, Siemens scored the best when participants were asked (1) what company will benefit most from increased lab spending, (2) have the best-in-class products, and (3) are best for personalized medicine (see summary for Exhibit 5).
Respondents said two of the major box providers, Siemens and Abbott, would benefit most from increases in lab spending; also ranking well were Bio-Rad and Becton. Siemens and Abbott are leading clinical chemistry and immunoassay providers, and Bio-Rad and Becton Dickinson have broad microbiology offerings.
You told us Siemens, followed by Bio-Rad, Gen-Probe, Abbott, and Johnson and Johnson, have best-in-class products. Regarding personalized medicine products, Siemens, followed by Cepheid, Illumina, LabCorp, Quest Diagnostics, and Roche, were well ranked. We were surprised by Illumina’s presence, given that respondents predominantly came from larger reference labs dominated by Siemens, Danaher, Johnson & Johnson, and Roche, and the lack of current diagnostic exposure for Illumina. (Ortho Clinical Diagnostics is a Johnson & Johnson company.) Cepheid also ranked exceptionally well considering the target audience.
Beyond the big box manufacturers in the clinical diagnostic lab (Siemens, J&J, Roche, and Abbott) stand (the included) companies Becton, Dickinson; Cepheid; Bio-Rad; and Thermo Fisher.
Bio-Rad and Becton, Dickinson may have scored well for spending and best in class due to their leading microbiology business, in addition to Becton, Dickinson’s solid cytology (SurePath), STD (ProbeTec and VIPER) and hospital-acquired infection (GeneOhm) franchises.
Oncology, as Expected, Greatest Benefit from Personalized Medicine
As expected, oncology is expected to see the greatest benefit from personalized medicine testing. Autoimmune/allergy was ranked second highest, somewhat surprisingly.
Exhibit 6: Which disease indication will benefit most from personalized medicine testing?
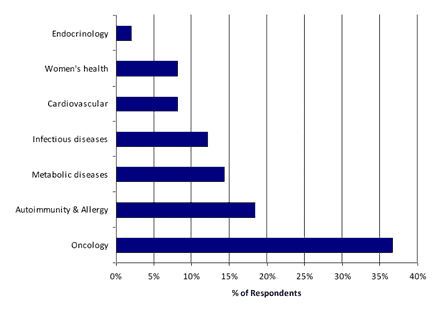
[Source: CLP and Mizuho Securities USA Survey]
Personalized Medicine Offers More Targeted Treatment
More targeted treatment, which could lead to greater efficacy and less side effects, was cited as the greatest benefit of personalized medicine testing. Increased efficacy and easier treatment decisions were the second most important reason.
Cost and insurance coverage were by far the greatest obstacles to increased adoption of personalized medicine testing, while lack of diagnostic performance was ranked lowest, suggesting the technology has advanced sufficiently.
Exhibit 7. What is the greatest benefit of personalized medicine?

[Source: CLP and Mizuho Securities USA Survey]
Molecular Tests Underpenetrated—HPV and Infectious Disease Testing Most Likely New Adds
The traditional clinical diagnostic test groups—hematology (94% of respondents), chemistry (87%), and immunoassays (83%), were used by the vast majority of respondents, unsurprisingly. Cardiovascular tests, including BNP and troponin (72%), also have broad usage, again unsurprisingly. Implementation of new tests appears a long-term growth driver—only 10% of respondents said they would be adding new tests in the next 12 months.
Molecular testing, despite growing at twice the industry, is still vastly underpenetrated, and complete implementation in clinical reference labs represents a long-term growth driver. HPV and infectious disease testing scored well for new tests that would be added in the next 12 months. HPV was only performed by approximately 28% of respondents, versus 94% for hematology tests, suggesting significant growth, and it also stood out as a test that would be added over the next 12 months. Chlamydia and gonorrhea (CT/NG) testing was only performed by 19% of respondents.
Exhibit 8. What is the greatest obstacle to increased adoption of personalized medicine tests?
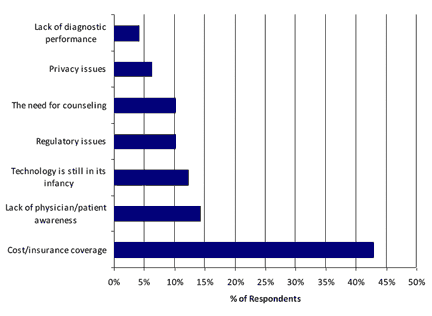
[Source: CLP and Mizuho Securities USA Survey]
HER2 Most-Used Personalized Diagnostic Test
Exhibit 9. What types of diagnostic tests do you use now and are likely to add over the next 12 months?
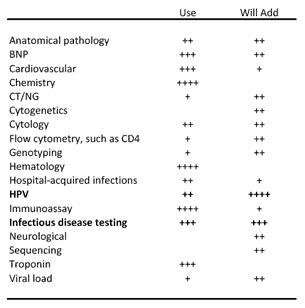
[Source: CLP and Mizuho Securities USA Survey]
Only 12% of respondents said they currently use personalized medicine tests, and only 18% said they were likely to add such tests, indicating the market still has substantial upside, but no single test stood out.
HER2 was the personalized medicine test used most often (see Exhibit 10), not surprising since it is one of the most prominent companion diagnostic tests, used with Herceptin.
MTHFR was the second-most used test, somewhat surprisingly.
Drug metabolism tests CYP2D6 and P450 ranked highest for tests that will be added in the next 12 months, along with cancer-related tests BRAF, KRAS, and UGT1A1.
Less than one-fourth of respondents indicated they use or would add personalized medicine tests. Therefore, compared to the broader survey, a lower level of significance has to be placed on this data. However, the data distinctly provides an excellent directional indicator.
Myriad’s Technical Leadership Displayed, Over and Above Patent Issues
Myriad Genetics has developed one of the leading personalized medicine franchises with its flagship test, BRACAnalysis (for BRCA gene mutations in the breast and ovarian cancer setting). There is currently a patent dispute involving BRCA patents between Myriad and the American Civil Liberties Union, focused on the ability to patent genes. The BRCA patents allow Myriad to be the only provider of BRCA testing. In order to gauge Myriad’s competitive position, we asked if labs would offer their own BRCA test, assuming no restrictions from patents.
Exhibit 10. Which personalized medicine tests do you currently use, and which ones will you add during the next 12 months?

[Source: CLP and Mizuho Securities USA Survey]
Ninety-three percent of those surveyed indicated they would not offer a rival test to Myriad’s BRCA test, even if patents were not an issue. Lack of technical capabilities was the key impediment to implementing BRCA testing.
The technical hurdles mitigate the intellectual property issue for Myriad and highlight the importance of Myriad’s extensive genetic variation and outcomes database associated with BRCA.
Exhibit 11. Would your lab make or offer a version of Myriad’s BRCA tests if the patents expired or were overturned (even though you wouldn’t have access to Myriad’s clinical outcomes and genetic databases)?
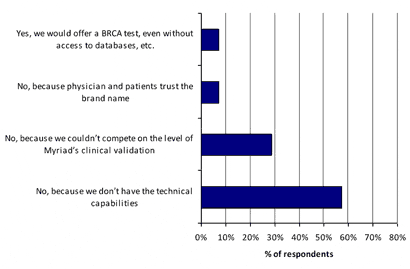
[Source: CLP and Mizuho Securities USA Survey]



