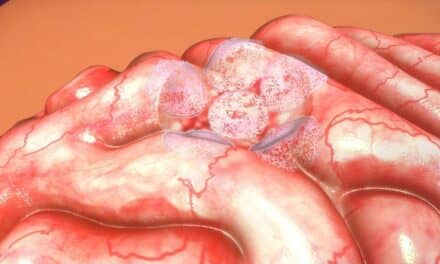
Approximately 450,000 Americans are currently being monitored for recurrent colorectal cancer. The presence of GC-C in the blood may be an early indicator of micrometastases that could escape detection by other current methods.
A collection kit with all of the components required for the collection and transport of patient blood specimens is available.
This facility is not a participating Medicare/Medicaid provider; the lab provides information required by insurance but does not bill directly for this test.
Targeted Diagnostics and Therapeutics
www.CLPmag.com
Keywords: reference lab, colorectal cancer