What do they really mean as the world yearns for a return to normal life?
By Fernando Chaves, MD
The covid-19 pandemic has impacted lives across all corners of the globe and we are all eager to go back to “normal.” But the hard truth is that, short of an effective vaccine being developed, return to normal life will need to happen in a tiered approach and with careful consideration of potential risks and benefits around decisions that not long ago were trivial. Key personal decisions will depend on people assessing their risk of getting sick and/or spreading the virus in the community, and for this reason a lot of attention is being devoted to antibodies and the serological tests used to detect them.
In this scenario, there are two critical open questions that can influence how large a role serological tests will play in the upcoming months as the world slowly moves to the next stage of the fight against this catastrophic pandemic:
• Do antibodies really provide immunity against covid-19?
• Are serological tests available today reliable enough to guide such critical decisions?
In this article, we will explore in detail the potential answers to these questions based on currently available information.
Do Antibodies Really Provide Immunity Against Covid-19?
Most likely yes. While data to fully establish this immunity as a scientifically documented fact has not yet been made available, and as additional research about this novel virus continues, there are several reasons and indirect evidence pointing in this direction. Long-term immunity is the most commonly observed response in the vast majority of human viral infections, including similar coronaviruses that caused previous outbreaks, including hepatitis A1and B1; poliomyelitis1; rotavirus1; measles1; mumps1; rubella1; varicella1; zoster1; human papilloma virus1; Middle Eastern respiratory syndrome2,3; and other previous coronavirus respiratory syndromes, such as SARS-CoV4; and Ebola.5

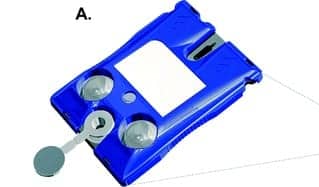
Figure 1: Paul Contestable, senior principal scientist with Ortho Clinical Diagnostics, holds Ortho’s Covid-19 antibody test, which runs on Ortho’s Vitros 3600 Immunodiagnostic System (pictured), as well as other Vitros systems.
Another coronavirus that caused previous outbreaks of severe acute respiratory syndrome, SARS-CoV, shares a considerable amount of its genetic material with SARS-CoV-2. Antibody testing showed that SARS-CoV immunity peaks at around 4 months and offers protection for roughly 2 to 3 years after the initial infection.6,7
Early reports during the current covid-19 pandemic demonstrated clinical improvement in severe covid-19 patients who received convalescent plasma, indicating that the antibodies produced by recovered patients likely had effective viral neutralization effect.8,9
A small initial animal study recently performed as part of SARS-CoV-2 vaccine development showed that a single dose of an investigational vaccine protected six rhesus macaques from pneumonia caused by the virus. The animals received the investigational vaccine 28 days before being infected with SARS-CoV-2 and were compared with three control animals that did not receive the vaccine. The vaccinated animals showed “no signs of virus replication in the lungs, significantly lower levels of respiratory disease and no lung damage compared to control animals.”10 While these results were generated in an animal model and in a small sample set, they do indicate that the antibodies generated after vaccine inoculation did confer protection when the animals were subsequently exposed to the virus.
Another small animal study infected two monkeys with SARS-CoV-2, waited until they developed and recovered from covid-19, and reexposed them to the virus 28 days after the initial infection.11 The reexposed monkeys showed a transient elevation of body temperature, but none of the other signs and symptoms of covid-19 as they had exhibited after the primary infection. The reexposed monkeys tested negative for the virus in nasopharyngeal and anal swabs, did not show lung abnormalities observed by X-ray, and upon a postmortem histological exam, did not show histopathological changes in lung tissue, nor any viral replication in any of the tissues examined.
Taken together, these findings are a strong indication that the previous exposure to SARS-CoV-2 provided protective immunity against reinfection with the same strain of the virus. An in vitro study showed that serum from a convalescent patient effectively prevented viral entry into the study cell lines,12 confirming the in vitro neutralizing efficacy of the antibodies triggered by SARS-CoV-2 infection. An in vitro neutralization study demonstrated that antibodies produced by eight study subjects inoculated with vaccine candidate mRNA-1273 (Moderna Therapeutics, Cambridge, Mass) were effective against live SARS-CoV-2.13
An in vitro neutralization study demonstrated that therapeutic antibody candidate STI-1499 (Sorrento Therapeutics, San Diego, Calif) yielded 100% inhibition of SARS-CoV-2 virus infection of healthy cells after 4 days of incubation. The antibody exhibited specific binding to the S1 subunit of the SARS-CoV-2 Spike protein and complete blockade of its interaction with ACE2 receptor.14 This study illustrates an efficient physiological mechanism for antibodies to grant immunity against infection.
Taken together, the findings above are strong indicators that patients who develop antibodies to SARS-CoV-2, through either previous infection or vaccination, will have effective protection against this virus, as is typically the case in viral infections. This assertion is critical as clinicians, healthcare institutions, governments, and even private individuals consider their options as we progress through the various stages of the ongoing covid-19 pandemic. It is also especially relevant as we consider the possible roles to be played by serological testing in critical decisions we will all have to make in the near future, not just from a public health/policy-making perspective, but also as individuals adjusting to life under the constant threat of this virus and waiting for a vaccine to finally become available. Ongoing studies are being conducted to confirm the protective value of antibodies as well as to establish the duration of immunity provided and whether antibody levels in the blood will impact this potential immunity.
Finally, in late April 2020, concerning reports came out of South Korea that several patients previously recovered from covid-19 had once again tested positive for the virus using molecular assays. These reports raised global fear that those were cases of reinfection that indicated an absence of long-term protection from antibodies produced during the initial infection. After weeks of investigation and contact tracing for these “repeat positive” individuals, the Korean Centers for Disease Control confirmed that those positive tests were due to lingering particles of the virus. No reinfection or transmission of the virus was documented in these individuals.15
Are Serological Tests Available Today Reliable Enough to Guide Such Critical Decisions?
Yes, but not all of them. In recent weeks and in response to the public calamity caused by covid-19, regulatory agencies worldwide including the FDA applied emergency protocols designed to facilitate rapid market access for products needed to respond to this crisis, among them serological tests for SARS-CoV-2. As a result, a multitude of tests are commercially available today, and not all of them have been through rigorous validation processes. Therefore, the performance of these tests, best measured as their diagnostic sensitivity and specificity, varies significantly. All stakeholders who rely on serological tests for critical decisions in this difficult time need to be educated about the exact performance of any assay they plan to use.
Serological assays vary in multiple dimensions, all of which have direct implications on the clinical utility of the test, including the antibody types being measured—IgG, IgM, IgA, or a combination of these; the performance of the test; and the viral protein targeted by the antibody being measured.
• Which antibodies does the test measure?
Tests that measure IgM or IgA, either alone or in combination with other antibodies, are ideal to aid in the diagnosis of SARS-CoV-2 infection because these are the first antibodies to appear in the blood when a patient is exposed to the virus.16,19,20 Some studies have shown IgA antibodies to be detectable in patient samples as early as 2 days after onset of symptoms, followed by IgM with a median time to seroconversion of 5 days. This information is critical for potential users considering the best option for serological assays intended to aid in the diagnosis of disease—assays which include IgA detection in their formulation are those best positioned to offer the highest rate of true positive results in recently infected patients.
• How does the test perform?
When considering tests for aid in diagnosis, potential users need to focus primarily on the sensitivity of the test, as provided in the instructions for use. Unfortunately, this critical marker of test performance varies tremendously from sensitivity levels as low as 61% at 14 days after onset of symptoms, to outstanding sensitivity levels with assays which offer 100% sensitivity as early as 8 days after onset of symptoms.17 Not surprisingly, the best sensitivity documented to date was observed with a “total” antibody assay which detects IgA, IgM, and IgG,17 thus reinforcing in real clinical use how critical IgA detection is to ensure optimal assay sensitivity and to make sure that infectious patients are not inadvertently released into the community.
It is critical to note that sensitivity levels must be compared taking into consideration how many days after symptom onset the samples were taken, because this is an acute infection and the timing of seroconversion varies among individuals. Therefore, even if an assay exhibited 100% sensitivity in its validation study, such an assay would have very limited clinical use in diagnosing covid-19 if this performance was validated with samples obtained too late in the disease progression.
Conversely, tests that measure IgG, either alone or in combination with other antibodies, can be used to identify individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection, because these are the antibodies which remain in circulation for longer periods of time and likely convey protection against reinfection, as discussed above.16
When considering tests for assessing immune response, based on this intended use, potential users need to focus primarily on the specificity of the test, as provided in the instructions for use. Unfortunately, this critical marker of test performance also varies significantly from specificity levels as low as 87.1%, to outstanding specificity levels with assays that offer 100% specificity.17
It is critical to note that even such an apparently small difference in specificity (100% to 98%) actually represents a major difference in the clinical value of the test in real life, due to the low prevalence of SARS-CoV-2 exposure in the general population. Even in Spain, one of the nations hardest hit by the pandemic, only about 5% of the population has been exposed to the virus.18 It is therefore reasonable to expect that current global levels of exposure are significantly lower.
If we assume a global population currently with 2% prevalence of exposure and we test this population with an assay with only 98% specificity (thus an assay which reports 2% false positives), we would end up with 2 out of every 4 positive tests reported (50%) being a false positive. This rate of erroneous results can be catastrophic as individuals may consider themselves protected when they were not.
• What viral protein does the test measure?
Finally, going back to the fundamental question at stake when it comes to the main value of serological testing in the fight against covid-19 (do antibodies really provide immunity against covid?), it is critical that potential users of serological tests be aware of the viral proteins targeted by the antibodies they will be detecting. It is the spike (S) protein of SARS-CoV-2 which facilitates viral entry into target cells. Entry depends on the S protein binding to the angiotensin-converting enzyme 2 (ACE2) as receptor and subsequently employing a cellular serine protease for S protein priming.12 Therefore, the antibodies which are truly relevant to establish whether a person is protected from SARS-CoV-2 infection are those against the S protein.
Most neutralizing antibodies against viral infection target surface proteins of the viruses, and anti-S1 or anti-S monoclonal antibodies have demonstrated neutralizing activities and prevented SARS-CoV-2 infection in cultured cells.14,21 Therefore, the S protein of SARS-CoV-2 is the target of almost all the vaccines currently under development,20 in which some initially published results were very encouraging. A DNA vaccine targeting S protein demonstrated protection from SARS-CoV-2 infection in nonhuman primates, and the vaccine-induced neutralizing antibody titers correlated with protective efficacy.22
Preliminary clinical results of the mRNA-1273 vaccine revealed effective induction of neutralizing antibodies in eight human subjects.13 All these observations suggested that anti-S or anti-S1 antibodies are not only a biomarker indicating viral exposure or infection, but also the active elements mediating protection or immunity. Indeed, antibody levels detected by serological tests targeting the S or S1 protein of SARS-CoV-2 often showed a strong correlation with neutralizing titers measured by neutralization tests.23
On the other hand, some of the currently available serological tests detect antibodies against other viral proteins, such as the nucleocapside N antigen. These tests are unlikely to directly detect neutralizing antibodies,17 thus raising additional questions about these assays as to the true value of a positive test result in indicating potential immunity against covid-19.
In summary, serological tests are a critical part of the global fight against covid-19 and can help determine global efforts to mitigate the social and economic consequences of this pandemic. One of the most critical potential uses of these tests is to establish whether an individual has been previously exposed to SARS-CoV-2, and as such is both immune from contracting the disease again and spreading it in the community.
While a categorical scientific confirmation that the antibodies detected by serological tests do convey this immunity is still pending, there are very strong reasons and indirect evidence pointing in this direction. And while the performance and clinical intended uses of various commercially available antibody tests vary greatly, there are some assays which offer the reliability needed to guide the critical decisions society and individuals will have to make. Stakeholders responsible for the selection of serological tests need to carefully educate themselves in the performance and other characteristics of the various options available to them.
Fernando Chaves, MD, is the global head of medical, clinical, and scientific affairs for Ortho Clinical Diagnostics. In this role, Chaves is responsible for clinical trials, new product development, and key opinion leader relationships. Chaves is a board-certified anatomical and clinical pathologist, with further certification in hematopathology.
REFERENCES
1. United States Centers for Disease Control. Immunization Schedules. https://www.cdc.gov/vaccines/schedules/index.html (May 21, 2020)
2. Shin HS, Kim Y, Kim G, et al. Responses to Middle East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis. 2019 Mar 5;68(6):984-992. doi: 10.1093/cid/ciy595.
3. Alshukairi AN, Zheng J, Zhao J, et al. High prevalence of MERS-CoV infection in camel workers in Saudi Arabia. mBio. 2018 Oct 30;9(5):e01985-18. doi: 10.1128/mBio.01985-18.
4. Lin Q, Zhu L, You L, et al. Duration of serum neutralizing antibodies for SARS-CoV-2: Lessons from SARS-CoV infection. J Microbiol Immunol Infect. 2020 Mar 25. doi: 10.1016/j.jmii.2020.03.015
5. Maruyama T, Rodriguez LL, Jahrling PB, et al. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J Virol. 1999 Jul;73(7):6024-30.
6. Wu L, Wang N, Chang Y, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007 Oct;13(10):1562-4. doi: 10.3201/eid1310.070576.
7. Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020 Apr 14;52(4):583-589. doi: 10.1016/j.immuni.2020.03.007.
8. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 Mar 27;323(16):1582-1589. doi: 10.1001/jama.2020.4783.
9. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of covid-19. J Clin Invest. 2020 Apr 7;138745. doi: 10.1172/JCI138745.
10. United States National Institutes of Health. News Releases. https://www.nih. gov/news-events/news-releases/investigational-chadox1-ncov-19-vaccine-protectsmonkeys-against-covid-19-pneumonia (May 21, 2020)
11. Bao L, Deng W, Gao H, et al. Lack of reinfection in rhesus macaques infected with SARS-CoV-2. Biorxiv. https://doi.org/10.1101/2020.03.13.990226.
12. Hoffmann M, Kleine-Weber H, Krüger N, et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. Biorxiv. https://doi.org/10.1101/2020.01.31.929042.
13. Moderna Therapeutics. Press Releases. https://investors.modernatx.com/newsreleases/news-release-details/moderna-announces-positive-interim-phase-1-data-itsmrna-vaccine (May 21, 2020).
14. Sorrento Therapeutics. News Releases. https://investors.sorrentotherapeutics. com/news-releases/news-release-details/sti-1499-potent-anti-sars-cov-2-antibodydemonstrates-ability (May 21, 2020).
15. Korean Centers for Disease Control. News Room. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid =0030 (May 21, 2020).
16. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020 May 6. doi: 10.1001/jama.2020.8259.
17. United States Food and Drug Administration. Emergency Use Authorizations. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/ emergency-use-authorizations (May 21, 2020).
18. Gobierno de Espana. Ministerio de la Salud. Estudio Nacional de Sero-Epidemiologia de la Infeccion por SARS-CoV-2 en Espana. Informe Preliminar 13 de Mayo de 2020. https://www.ciencia.gob.es/stfls/MICINN/Ministerio/FICHEROS/ENECOVID_Informe_ preliminar_cierre_primera_ronda_13Mayo2020.pdf (May 21, 2020).
19. Yu H, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020 May 12;2001526. doi: 10.1183/13993003.01526-2020.
20. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020 Mar 21;ciaa310. doi: 10.1093/cid/ciaa310.
21. Wu Y, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274-1278. doi: 10.1126/science.abc2241
22. Yu J, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. Epub. May 20, 2020. doi: 10.1126/science.abc6284 (2020).
23. Ni L, et al. Detection of SARS-CoV-2-specific humoral and cellular 2 immunity in COVID-19 convalescent individuals. Immunity. March 2020.