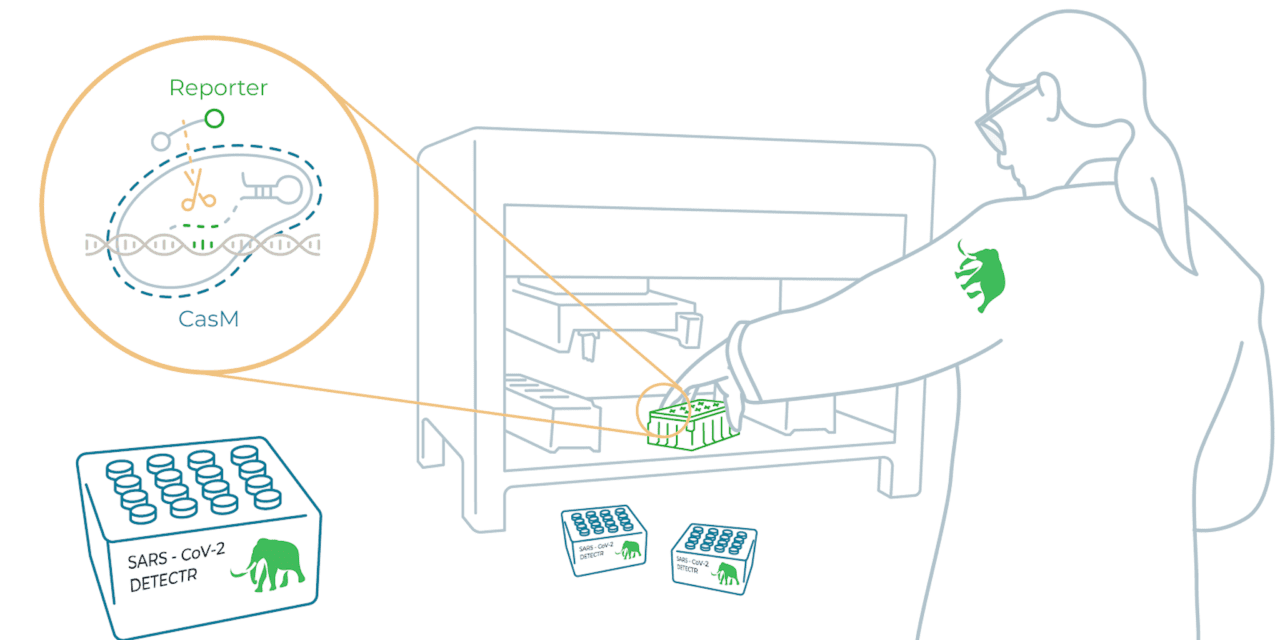
Mammoth Biosciences, Brisbane, Calif, now has a co-marketing agreement with Agilent Technologies, Santa Clara, Calif, to support the anticipated launch of a complete CRISPR-based SARS-CoV-2 diagnostic solution comprising Agilent’s Bravo automation workstation and Mammoth Biosciences’ Detectr Boost assay. The partnership marks Agilent’s commitment to be a prominent supplier of solutions for the covid-19 and infectious disease diagnostics market.
Together, the Detectr Boost SARS-CoV-2 Kit and Agilent automated liquid handling system would provide a complete workflow for high-throughput covid-19 testing—a turnkey, sample-to-answer solution for commercial laboratories that enables a multi-fold increase in testing capacity compared to most currently available solutions, while ensuring highly accurate and sensitive results required for SARS-CoV-2 diagnostic testing. The assay and automation solution will leverage Agilent’s Bravo Liquid Handling System, including the Bravo BenchCel DB Workstation and BioTek plate readers, predicted to deliver a workflow capable of performing over 4,000 covid-19 tests per day.
Read Mammoth Biosciences Gets NIH Contract to Scale CRISPR Covid-19 Diagnostic Platform
“Mammoth’s mission is to address challenges across healthcare by harnessing the full potential of the CRISPR platform to read and write the code of life,” says Trevor Martin, co-founder and chief executive officer at Mammoth Biosciences. “This partnership will help address the need for more widespread testing options for covid-19, helping to fill the gap in the market as testing labs run into supply issues or reach capacity.”
“A highly-automated workstation for SARS-CoV-2 testing provides the capacity needed to bring routine, robust testing to the broader market,” says David Edwards, associate vice president for marketing in the Agilent mass spectrometry division. “By partnering with Mammoth Biosciences, we will be able to provide a simplified workflow that addresses the specific needs of high-throughput clinical testing laboratories. Agilent is honored to contribute in the fight to curtail the impact of covid-19 alongside the global scientific community.”
With its recent acquisition of BioTek, Agilent is uniquely positioned to support the multi-discipline, multi-factorial efforts required to make an impact in addressing complex human diseases. Whether through the development of tests and instruments or as a desired partner for co-development efforts, Agilent continues to leverage its solutions and expertise to drive progress in research, test development, community surveillance, and vaccine and drug development.
The Mammoth high-throughput CRISPR-based Detectr Boost SARS CoV-2 platform,is expected to be submitted for FDA EUA soon.
This project is supported by the NIH Rapid Acceleration of Diagnostics (RADx) program and has been funded in whole or in part with Federal funds from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Department of Health and Human Services.
For more information, visit Mammoth Biosciences and Agilent Technologies.