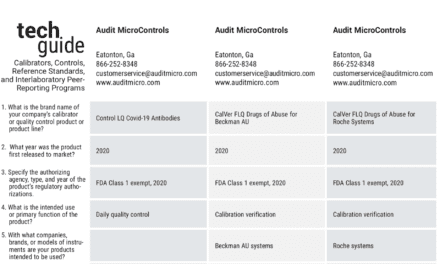
LGC Maine Standards, Cumberland Foreside, ME, has announced the addition of cerebrospinal fluid total protein to its Validate body fluids kit, enhancing the only comprehensive body fluids kit on the market. The addition will facilitate validation of laboratory-developed tests, documentation of linearity and calibration verification, and verification of the reportable range.
Adding cerebrospinal fluid total protein (CSF-TP) enhances the available analytes in the company’s simulated body fluid matrix, which now includes albumin, amylase, cancer antigen 19-9, carcinoembryonic antigen, cholesterol, creatinine, glucose, lactate, lactate dehydrogenase, total protein, triglycerides, and urea nitrogen.
Each Validate body fluids kit is liquid and ready-to-use and prepared using the CLSI EP06-A ‘equal delta’ method of sample preparation. The kit offers five distinct concentrations to establish the reportable range. Users dispense the solution from each dropper bottle directly into five sample cups, and run in replicates.
Release of the Validate body fluids kit enables clinical laboratories to complete their required verification of body fluids linearity and calibration, and validation of laboratory-developed tests for body fluid analytes—establishing the reportable range while minimizing manual dilutions. While augmenting daily QC, use of the product assists with fulfilling various quality control requirements—such as verification of the analytical measurement range and reportable range for linearity and calibration—under domestic and international regulations and accreditation schemes.
Validate linearity and calibration verification kits are currently available for more than 130 analytes, including anemia, body fluids. cardiac markers, enzymes, fertility, general chemistries, HbA1c, hemostasis, lipids, serum proteins, therapeutic drugs, thyroids, tumor markers, urine chemistries, vitamin D, and whole blood glucose.
For further information, visit LGC Maine Standards.