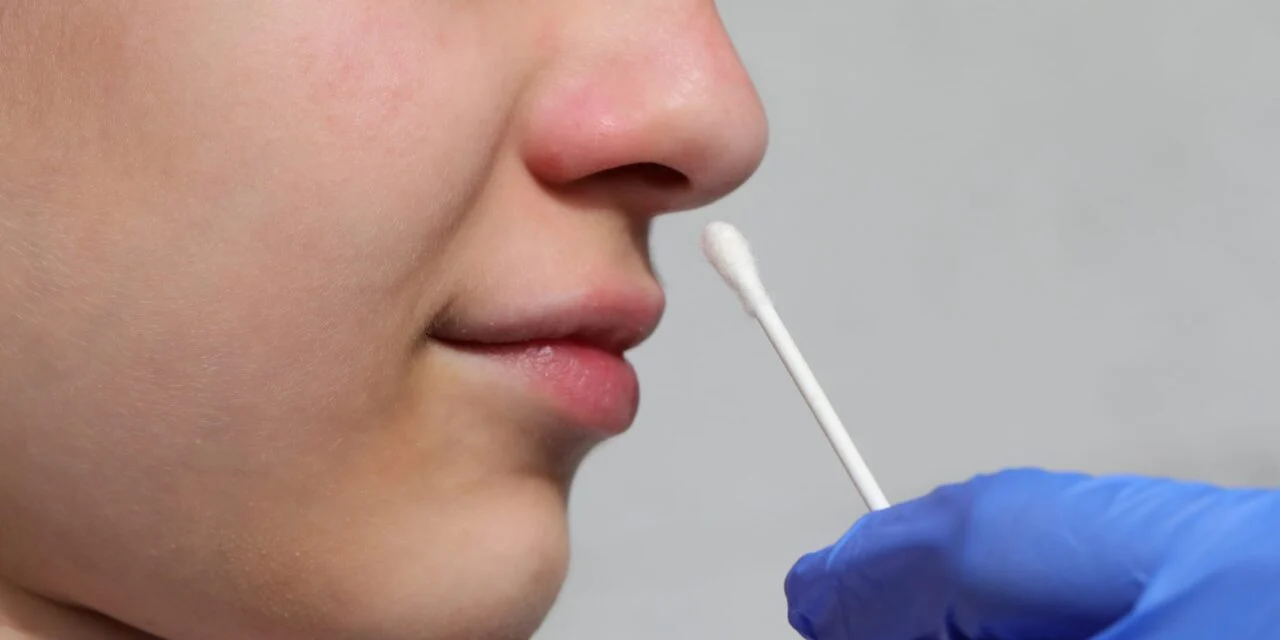
The French diagnostics company announced the clearance allows healthcare professionals to collect specimens by swabbing only the anterior part of the nasal cavity.
bioMérieux has received US Food and Drug Administration (FDA) 510(k) clearance and CLIA waiver for anterior nasal swab specimens as an additional sample type for use with the Biofire Spotfire Respiratory/Sore Throat Panel Mini, specifically for respiratory testing applications.
The French diagnostics company announced the clearance allows healthcare professionals to collect specimens by swabbing only the anterior part of the nasal cavity, providing significantly more comfort for patients compared to traditional nasopharyngeal swabs while maintaining comparable reliability.
The Biofire Spotfire R/ST Panel Mini, launched in the US in 2024, is a single multiplex PCR test that detects five of the most common viral and bacterial causes of respiratory or sore throat infections in approximately 15 minutes. The platform previously offered nasopharyngeal swab and throat swab collection methods.
“Today, beyond the accuracy of diagnostic results, patients seek greater comfort in testing. The COVID-19 pandemic has especially underscored the importance of patient well-being in healthcare settings,” says Dr Charles K Cooper, executive vice president and chief medical officer at bioMérieux, in a release. “The opportunity to provide the same quality and performance with a less invasive sample with our Biofire Spotfire R/ST Panel Mini truly illustrates our mission to make an impact on patients’ health.”
Expanding Point-of-Care Testing Options
The clearance addresses growing demand for decentralized diagnostic testing that emerged during the COVID-19 pandemic. Point-of-care testing brings diagnostic solutions closer to patients, potentially reducing time-to-result for identifying infectious diseases and determining appropriate treatment protocols.
Anterior nasal swab collection has gained recognition for ease of collection and superior patient comfort. Research indicates that nasal swabs demonstrate comparable reliability to nasopharyngeal swabs for molecular detection of respiratory pathogens.
The respiratory test menu detects human rhinovirus, influenza A virus, influenza B virus, respiratory syncytial virus, and SARS-CoV-2. The sore throat menu specifically tests for Streptococcus pyogenes.
“Anterior Nasal Swab specimen collection clearance on the Biofire Spotfire R/ST Panel Mini marks another milestone in bioMérieux’s Point-of-Care offer and strengthens our presence in this market segment,” says Jennifer Zinn, executive vice president of clinical operations at bioMérieux, in a release.
Market Availability
The additional anterior nasal swab specimen type for the BIOFIRE SPOTFIRE R/ST Panel Mini will be available for use in the US before the respiratory season in the third quarter of 2025.
The CLIA waiver designation allows the test to be performed in settings with less stringent laboratory oversight requirements, expanding access to point-of-care respiratory testing in various healthcare environments.
ID 244766466 © Fedecandoniphoto | Dreamstime.com