To analyze the tumor microenvironment affecting cancer patients, understanding the spatial organization of multiple biomarkers present on a tissue section is important. Staining and visualizing several targets simultaneously is possible with a new technique known as ‘multiplexed immunofluorescence’—the fluorescent staining of multiple biomarkers on the same tissue sample. A recent study has provided a proof of concept for the use of microfluidics in the automation of such high-multiplexed immunostaining.1
Unlike singleplex immunostaining techniques, multiplexed staining enables researchers to perform quantitative analysis of a variety of factors, such as identifying correlations among several cell families, or determining the activation status of cell populations within the tumor microenvironment.
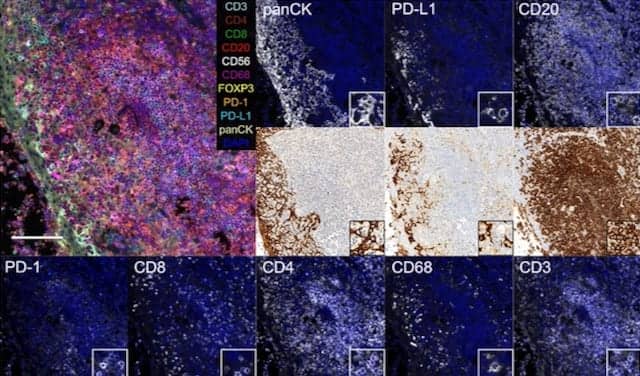
The recent study illustrated the potential of Lunaphore’s microfluidic staining device to stain 10 markers relevant to immunooncology (CD3, CD4, CD8, CD20, CD56, CD68, CK, FOXP3, PD-1, and PD-L1) in a sequential immunofluorescence protocol consisting of cycles of staining, imaging, and elution of antibodies. The study also demonstrated a quantitative approach to the automation of both experimental and analytical methods using the Lunaphore system.
Lunaphore’s automated device is based on fast fluidic exchange technology, utilizing a microfluidic tissue processor capable of staining each marker within 9 to 11 minutes. The staining module of the company’s instrument employs a microfluidic chip, called a ‘look-through’ chip, that is equipped with an imaging window. The imaging system is integrated within the device, and can gather images through the chip in order to fully automate the staining–imaging process.
The study also explored the use of high-quality image processing algorithms to map each cell in the tissue, identifying the coexpression and colocalization patterns of biomarkers in order to classify immune cells and their activation status.
“Combining the rapid immunofluorescence staining achieved with our microfluidic technology, together with an integrated microscopy system, we are able to perform high-plex assays using off-the-shelf unlabeled primary antibodies in unprecedented times,” says Diego Dupouy, chief technology officer at Lunaphore. “We are currently developing a high-throughput instrument to run 40-plex assays in a fully automated manner, using the method demonstrated in this study.”
Incorporating microfluidics into a microscope-integrated multistaining instrument offers several advantages:
- It is possible to automate high-multiplexing protocols.
- The number of markers stained can be very high. The recent study demonstrated the automation of 10-plex processing, but the system permits users to stain up to 40 markers on a single sample.
- The use of standard unlabeled primary antibodies will prevent issues related to availability, which often occur with the use of labeled antibodies that are required by other multistaining techniques.
For more information, visit Lunaphore Technologies.
Reference
- Migliozzi D, Pelz B, Dupouy DG, Leblond AL, Soltermann A, Gijs MAM. Microfluidics-assisted multiplexed biomarker detection for in situ mapping of immune cells in tumor sections. Microsyst Nanoeng. 2019;5:59; doi: 10.1038/s41378-019-0104-z.
Featured image:
Fluorescence images of biomarkers in tonsils from microfluidic 10-plex immunofluorescence and bright-field images of conventional single-plex immunohistochemistry on adjacent slides. Scale bars: 100 µm (overview images) and 15 µm (insets).