Greater access to nucleic acid amplification tests is needed to address rising rates of extragenital chlamydia and gonorrhea
By Valentina Schmidt, PhD
Microorganisms and the infectious diseases they cause have been a threat to mankind’s existence throughout the history of human civilization. Century after century, epidemics have been leaving their marks on the human race, changing the course of human history in their wake. While advances in biomedical science have ensured our survival, the threat of infectious diseases still remains today.
The incidence of sexually transmitted infections (STIs) is at an epidemic level. According to the US Centers for Disease Control and Prevention (CDC), more than 19.7 million new STI cases are diagnosed in the United States each year.1 These include chlamydia, gonorrhea, syphilis, and trichomoniasis, as well as viral infections such as hepatitis B virus (HBV), herpes simplex virus-2 (HSV-2), human immunodeficiency virus (HIV), and human papillomavirus (HPV). CDC estimates the current nationwide prevalence of STIs among both men and women to be more than 110 million cases.1
Chlamydia, caused by the bacterium Chlamydia trachomatis (CT), and gonorrhea, caused by the bacterium Neisseria gonorrhoeae (NG), are the two most frequently reported communicable diseases in New York State and nationwide. Both infections are linked to various serious long-term health problems, including an increased risk of acquisition and transmission of HIV.2
CDC analyses presented at the biennial National STD Prevention Conference in August 2018 reported alarmingly steep and sustained increases in sexually transmitted diseases (STDs) throughout the United States.2 For 2017, CDC documented nearly 2.3 million cases of chlamydia, gonorrhea, and syphilis combined, exceeding the previous record of 2016 by more than 200,000 cases, and marking the fourth consecutive year of sharp increases in these STDs. From 2013 to 2017, gonorrhea diagnoses increased 67% overall (from 333,004 to 555,608 cases) and nearly doubled among men (from 169,130 to 322,169). The increase in gonorrhea diagnoses among women is also evident for the third year in a row (from 197,499 to 232,587). Chlamydia remains the most common condition reported to CDC. More than 1.7 million cases were diagnosed in 2017, with 45% among females aged 15 to 24.
While youth aged 15 to 24 represent just 24% of the sexually experienced population in the United States, they are disproportionately affected by STIs, accounting for approximately 50% of all new cases. Gender-wise, the annual number of new infections nationwide is close to equal among young women and young men, 49% versus 51%, respectively.
Geographically, rates of STIs differ by state. In 2012, for example, chlamydia rates per 100,000 people ranged from 233 cases in New Hampshire, to 774 in Mississippi, and 1,102 in the District of Columbia.2
The State of New York has one of the highest rates of chlamydia and syphilis in the country. According to CDC data for 2017, New York ranked 9th, 21st, and 6th among all states for the total number of diagnoses of chlamydia, gonorrhea, and syphilis, respectively.3 Demographically, young people, gay, bisexual, and other men who have sex with men (MSM) have the highest rates of STIs in New York. Chlamydia continued to be the most common reportable STI in New York in 2017 with 116,843 diagnoses, a 7% increase over 2016 and the highest number of diagnoses since chlamydia became reportable in 2000. The highest rates for chlamydia were among females aged 15 to 24. Gonorrhea diagnoses increased 17% in 2017, to 34,111 total reported diagnoses. The rate of increase among males was higher than among females (21% compared to 11%). By age group, rates were highest among males aged 20 to 29, and among females aged 15 to 24.
Viewed together, such disturbing statistics highlight the continuing need for new technologies that can provide rapid, accurate, and cost-effective detection and diagnosis of STIs.
Extragenital STIs
The incidence of STIs at extragenital sites, including the oropharynx and rectum, is also on the rise, especially for chlamydia and gonorrhea (Figure 1). For the first time since 2006, gonorrhea has become more prevalent among men than women. From 2010 to 2014, the number of gonorrhea cases among men increased by 27.9%. This increase is most likely attributed to increased diagnoses among gay, bisexual, and other men who have sex with men (MSM).5,6 A recent review of studies reporting the prevalence of extragenital chlamydia and gonorrhea in women, men who have sex only with women (MSW), and MSM indicated the changing prevalence of extragenital STIs among these groups.7
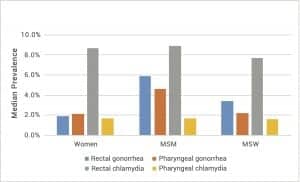
Figure 1. Comparison of median prevalence of extragenital chlamydia and gonorrhea in specific populations nationwide.
CDC’s STD surveillance network encompasses 42 STD clinics across the United States. Data from the network, based on screening results from 21,994 MSM, indicates the rising prevalence of infection for pharyngeal chlamydia (2.9%), pharyngeal gonorrhea (7.9%), rectal chlamydia (14.1%), and rectal gonorrhea (10.2%) in this population.8
These numbers are in some concordance with the extragenital chlamydia and gonorrhea rates determined as part of the national HIV behavioral surveillance study conducted at MSM-frequented venues in five US cities (Houston, Miami, New York, San Francisco, and Washington, DC). Overall, 13.3% of the 2,075 participants were infected with at least one of the two STDs at one or two anatomic sites. Prevalence of rectal chlamydia (7.3%) was higher than that of rectal gonorrhea (4.5%), whereas prevalence of pharyngeal gonorrhea (4.6%) was higher than that of pharyngeal chlamydia (1.4%).9
As the majority of extragenital chlamydia and gonorrhea infections are asymptomatic, more than 70% of such infections would be missed with urogenital screening alone.10 Among MSM with extragenital infections, it has been reported that only 5.1% of pharyngeal and 11.9% of rectal infections were symptomatic. The most common pharyngeal symptoms were pharyngitis, localized lymphadenopathy, and inflammation of the oral cavity; rectal symptoms may include pruritus, anal discharge, burning, inflammation, pain, and erythema around the anus.11 A genital-only screening would miss a major portion of such extragenital STIs (Figure 2).12
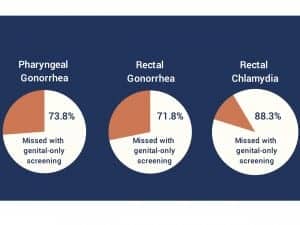
Figure 2. Percentage of extragenital sexually transmitted infections missed with genital-only screening.12
The overwhelming evidence from such studies indicates a high prevalence of extragenital chlamydia and gonorrhea infections among MSM, the asymptomatic nature of most such infections, and the high frequency of extragenital infection without concurrent urogenital infection—all of which support the need for routine screening at extragenital sites.
The frequency of screening should be linked to risk behaviors. Current CDC guidelines for sexually active MSM recommend an annual screening for STDs at all anatomic sites of exposure regardless of condom use. For MSM at elevated risk for STDs, CDC guidelines call for more frequent screening (eg, every 3 to 6 months). And among MSM on preexposure prophylaxis (PrEP) for HIV, the guidelines call for screening every 3 months.13
Current STI Testing Technologies
The multiple testing technologies currently available to clinical laboratories for the direct detection of STI pathogens can be divided into two general categories: culture and nonculture. In accord with their capabilities and performance characteristics, not all of the existing methods are recommended for routine diagnostic use.
Microbiological culture methods represent the oldest reference standards in the field, but they are time-consuming, difficult to standardize, and not readily amenable to automation, transport, and storage. Yet it is important for clinical laboratories to maintain their capability to culture both C. trachomatis and N. gonorrhoeae in order to perform a test of cure in suspected cases of treatment failure, to monitor changes in antibiotic susceptibility, and to detect rare infections caused by variant or mutated strains.14
Among the nonculture STI tests available, CDC does not recommend the use of enzyme immunoassays or genetic transformation tests because of their lack of precision.
By contrast, nucleic acid amplification tests have become the most widely adopted methods for STI laboratory screening.15 From a technology standpoint, commercial nucleic acid amplification tests differ in the methods they use to amplify target nucleic acid sequences. Common amplification methods include polymerase chain reaction (PCR; Abbott RealTime, Roche Cobas), strand displacement amplification (BD ProbeTec ET system), and transcription-mediated amplification (Hologic Aptima Combo 2 assay). Each of these methods has distinct advantages and disadvantages.16
CDC recommends nucleic acid amplification tests as the current gold standard for the detection of C. trachomatis and N. gonorrhoeae.17 The technologies related to nucleic acid amplification tests have undergone immense improvements since 2002, resulting in expanded screening using less-invasive specimen collection and reduced assay costs.18 The performance characteristics of nucleic acid amplification tests—including their speed, overall sensitivity, specificity, precision, ease of automation, and compatibility with specimen transport methods—are superior to those of any other test type available for the diagnosis of chlamydial and gonococcal infections.17
Most commercial nucleic acid amplification tests have received FDA clearance for the detection of C. trachomatis and N. gonorrhoeae in endocervical, vaginal, urethral, and urine specimens. In May 2019, FDA cleared the first two such tests for the detection of C. trachomatis and N. gonorrhoeae in extragenital specimens: the Xpert CT/NG assay from Cepheid, Sunnyvale, Calif; and the Aptima Combo 2 assay from Hologic, Marlborough, Mass.

Figure 3. The Enzo Molecular Diagnostics open platform has been developed to automate the workflow for nucleic acid amplification testing at Enzo Clinical Labs.
The LDT Approach
Enzo Clinical Labs, a subsidiary of Enzo Biochem, Farmingdale, NY, has extensive experience with both FDA-cleared diagnostics and laboratory-developed tests (LDTs) for women’s health targets, including STIs (Figure 3). To meet the rising incidence of such infections in its home state and beyond, Enzo Clinical Labs has identified the need for cost-saving options, including wider availability of LDTs. Before using their LDTs with clinical samples, however, labs must perform diligent in-house validation of their tests to meet the requirements of the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and, in New York State, the standards of the clinical laboratory evaluation program of the New York State Department of Health (NYSDOH).
According to the NYSDOH website, increasing laboratory capacity to perform extragenital chlamydia and gonorrhea testing is critical for reducing the burden of chlamydia and gonorrhea infections, interrupting STI transmission, and reducing the risk of HIV transmission.19 This strategy is also aligned with Governor Andrew M. Cuomo’s ‘Ending the Epidemic’ initiative. Nevertheless, only 13 diagnostic laboratories in New York State are currently approved to perform extragenital nucleic acid amplification testing for chlamydia and gonorrhea.
To increase the availability of extragenital nucleic acid amplification testing services for public health agencies in regions of New York State that are currently underserved, NYSDOH has recently contracted Enzo Clinical Labs to validate its laboratory-developed Ampiprobe CTNGTV PCR assay for use with pharyngeal and rectal specimens.
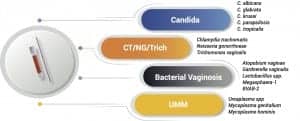
Figure 4. Ampiprobe, a multiplex nucleic acid amplification technology, provides a time- and cost-efficient solution for multiple-pathogen testing for sexually transmitted infections, using a single sample to detect 16 pathogens.
The Ampiprobe CTNGTV PCR assay is a multiplex nucleic acid amplification test developed by Enzo Life Sciences, another subsidiary of Enzo Biochem, and conditionally approved by NYSDOH’s clinical laboratory evaluation program for detection of C. trachomatis, N. gonorrhoeae, and T. vaginalis DNA in vaginal swab specimens (Figure 4). The assay utilizes Ampiprobe technology, which incorporates the probe method into primer design and enables the use of real-time PCR detection.20 The assay’s use of proprietary fluorescence-labeled and quencher-labeled paired primers allows for real-time detection of PCR product by monitoring the emission intensity of the fluorescent reporter dyes released or, more specifically, quenched, during the amplification process.
Enzo’s validation study has demonstrated the high overall accuracy and precision of the Ampiprobe CTNGTV assay in both pharyngeal and rectal specimen types. A low limit of detection, fast turnaround time, affordability, and ease of use distinguish the Ampiprobe assay from other commercial nucleic acid amplification tests in the infectious disease space.
Conclusion
In the quest to stop the current STI epidemic, it is imperative that healthcare professionals have access to rapid, sensitive, and cost-effective molecular assays utilizing cutting-edge technologies for the detection of C. trachomatis and N. gonorrhoeae in a broad range of specimen types. This need is especially critical in the current climate of cuts in healthcare, decreased reimbursement for laboratory services, and rising operational costs.
Public health authorities and other healthcare professionals are already taking necessary steps to support increased preventive measures and public education about the STI crisis. In addition to such initiatives, the introduction of an innovative, multiplex, low-cost nucleic acid amplification test compatible with urogenitial and extragenital collection devices will also play an important role in helping to identify and treat chlamydia and gonorrhea infections in high-risk and underserved communities, both in New York State and nationwide.
Valentina Schmidt, PhD, is a translational scientist at Enzo Clinical Labs. For further information, contact CLP chief editor Steve Halasey via [email protected].
REFERENCES
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193; doi: 10.1097/OLQ.0b013e318286bb53.
- Sexually Transmitted Disease Surveillance, 2017. Atlanta: Centers for Disease Control and Prevention, 2018; doi: 10.15620/cdc.59237.
- Sexually Transmitted Infections: Surveillance Report. Albany, NY: New York State Department of Health, 2017. Available at www.health.ny.gov/statistics/diseases/communicable/std/docs/sti_surveillance_report_2017.pdf. Accessed June 13, 2019.
- Owusu-Edusei K Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40(3):197–201; doi: 10.1097/OLQ.0b013e318285c6d2.
- Fox KK, del Rio C, Holmes KK, et al. Gonorrhea in the HIV era: a reversal in trends among men who have sex with men. Am J Public Health. 2001;91(6): 959–964.
- Rietmeijer CA, Patnaik JL, Judson FN, Douglas JM Jr. Increases in gonorrhea and sexual risk behaviors among men who have sex with men: a 12-year trend analysis at the Denver Metro Health Clinic. Sex Transm Dis. 2003;30(7):562–567.
- Chan PA, Robinette A, Montgomery M, et al. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Inf Dis in Obstet Gynecol. 2016;2016:5758387; doi: 10.1155/2016/5758387.
- Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men: STD Surveillance Network, United States, 2010–2012. Clin Infect Dis. 2014;58(11):1564–1570; doi: 10.1093/cid/ciu184.
- Johnson Jones ML, Chapin-Bardales J, Bizune D, et al. Extragenital chlamydia and gonorrhea among community venue-attending men who have sex with men, five cities, United States, 2017. MMWR Morb Mortal Wkly Rep.2019;68(14):321–325; doi: 10.15585/mmwr.mm6814a1.
- Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Inf Dis. 2005;41(1):67–74; doi: 10.1086/430704.
- Dudareva-Vizule S, Haar K, Sailer A, et al. Prevalence of pharyngeal and rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among men who have sex with men in Germany. Sex Transm Infect. 2014; 90(1):46–51; doi: 10.1136/sextrans-2012-050929.
- Simple ways to make the health of gay men a priority [infographic, online]. Washington, DC: Gay Men’s Health Equity Work Group, n.d. Available at: www.nastad.org/sites/default/files/Uploads/GMHE-Infographic/gmhe-infographic-1.pdf. Accessed June 13, 2019.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep.2015;64(RR-03): 1–137.
- Won H, Ramachandran P, Steece R, et al. Is there evidence of the new variant Chlamydia trachomatis in the United States? Sex Transm Dis. 2013;40(5):352–353; doi: 10.1097/olq.0b013e3182841786.
- Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49(10):3610–3615; doi: 10.1128/jcm.01217-11.
- Rönn MM, Mc Grath-Lone L, Davies B, Wilson JD, Ward H. Evaluation of the performance of nucleic acid amplification tests (NAATs) in detection of chlamydia and gonorrhoea infection in vaginal specimens relative to patient infection status: a systematic review. BMJ Open. 2019;9(1):e022510; doi: 10.1136/bmjopen-2018-022510.
- Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae, 2014. MMWR Recomm Rep. 2014;63(RR-02):1–19.
- Johnson RE, Newhall WJ, Papp JR, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeaeinfections, 2002. MMWR Recomm Rep. 2002;51(RR-15):1–38.
- Prevention agenda 2019–2024: New York State’s health improvement plan [online]. Albany, NY: New York State Department of Health, 2019. Available at: www.health.ny.gov/prevention/prevention_agenda/2019-2024. Accessed June 13, 2019.
- Hauser W, Wang G, Schapfel D. Comprehensive women’s health diagnostic testing using innovative multiplex PCR assays [abstract ID41, online]. Poster presented at the annual meeting of the Association for Molecular Pathology, Salt Lake City, November 16–18, 2017. J Mol Diagn. 2017;19(6):982; doi: 10.1016/s1525-1578(17)30482-8.
Featured image:
Gonorrhea. Microphotograph courtesy Centers for Disease Control and Prevention public health image library (ID 6511).