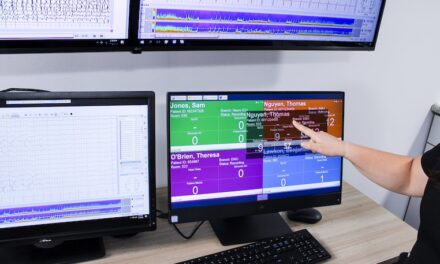
When the Human Genome Project was completed in 2003, it took 13 years and $2.7 billion to sequence the 30,000 genes that made up the human genome. At the end of the project, the National Human Genome Research Institute (NHGRI) talked about the exciting advancements that this understanding could create. They believed at the time that novel drug therapies were 10 to 15 years away, but said we would have “the ability to predict individual future health risks, and the ability to implement an enhanced approach to preventive medicine. In the next decade, we may also be better able to determine which drugs work best for individuals, based on their genetic makeup.”
Since 2003, according to Trish Meek, director of strategy, Life Sciences, Thermo Fisher Scientific, Philadelphia, informatics technology such as next-generation sequencing has accelerated the development of novel therapies. Several cancer therapies already exist that target specific alterations in cancer-related genes: Trastuzumab (marketed as Herceptin), which targets breast tumors that overexpress the HER2/neu protein, is perhaps the best known; and other approved therapies or those in late-stage development target various alterations in major cancer genes, including EGFR (lung, colorectal, and pancreatic cancers), KRAS (leukemia, pancreatic, colorectal, and lung cancer), and BRAF (melanoma, and potentially several other cancers). It is unlikely that anyone would have predicted that by 2011 a person could have their entire genome sequenced and interpreted in less than a month for a few thousand dollars. The reduction in cost and time for genetic sequencing has made molecular diagnostics—genetic testing used for clinical diagnostic purposes—a reality, and the industry is expected to reach $5.8 billion by 2015, Meek says.
NEXT-GENERATION TOOLS
Through molecular diagnostic testing, physicians are able to diagnose and treat their patients based on their unique genetic makeup. This more personalized approach to medicine has also changed the way clinical research is done and opened the doors to what is now called translational research; that is, bringing the results from the clinical lab directly to the patient’s bedside. Two next-generation laboratory and patient-management tools that are instrumental in making this a reality are laboratory information management systems (LIMS) and laboratory information systems (LIS). Thermo Fisher cites Bruce A. Friedman, MD, active emeritus professor of pathology at the University of Michigan Medical School and a vocal commentator on the role informatics plays in medicine: “One of the major goals of the translational research movement has been to bridge the gap from bench to bedside, and any initiative to blend the LIS with the LIMS can be understood as an extension of this quest. The lesson from all of this is obvious. We now need to make an effort to understand the relative strengths of LIS and LIMS, and proceed to develop new systems that capture the best features of each of them.”

Dave Champagne, vice president and general manager, Informatics, Thermo Fisher Scientific

Trish Meek, director of strategy, Life Sciences, Thermo Fisher Scientific
This drive to deliver personalized medicine has been a catalyst for a more symbiotic relationship between patient-centric processes in the clinic and the sample-centric lab environment. The ultimate goal of this new symbiosis is a streamlined end-to-end information flow following the patient from the point of care, to molecular testing and results analysis, to diagnosis and treatment. “To make personalized cancer medicine a reality, life science and information technology have to evolve in tandem,” says Dave Champagne, vice president and general manager, Informatics, Thermo Fisher Scientific, Philadelphia. “Routine molecular diagnostics requires a convergence of the up-to-now separate systems that have managed work in the lab (the LIMS) and the clinic (the LIS). The industry is asking for, and the science is requiring, a single lab-centric solution that delivers patient-centric results.”
Due to the patient-specific nature of the testing, labs must have in place all of the tools and compliant methodologies that are required to support Clinical Laboratory Improvement Amendments (CLIA) and Health Insurance Portability and Accountability Act (HIPAA) regulations. Labs also need to manage complex sample-oriented workflows (including sophisticated instruments and automation) and support business processes. The new challenge for clinical testing labs responding to the increase in genetics testing is bringing together the functionality of informatics systems that manage patient information and those that manage lab and sample data.
FILLING THE GAPS WITH A NEW CLINICAL LIMS
Thermo Fisher, in partnership with iConnect Consulting, San Francisco, has developed a new clinical LIMS that combines the research-centered functionality of a traditional LIMS with capabilities typically found in LIS, which manage patient records, administrative functions, and privacy compliance in hospitals and clinical settings. “We have been exposed to a number of LIMS/LIS products and noticed major functional gaps preventing those products from providing a complete end-to-end solution to a modern molecular diagnostic laboratory,” says Andrew Sinyaver, president of iConnect Consulting. Sinyaver says iConnect was happy to partner with Thermo Fisher Scientific, which understood the challenges facing the clinical market and could leverage its informatics platform to “bridge the gaps and develop a complete Clinical LIMS solution.”
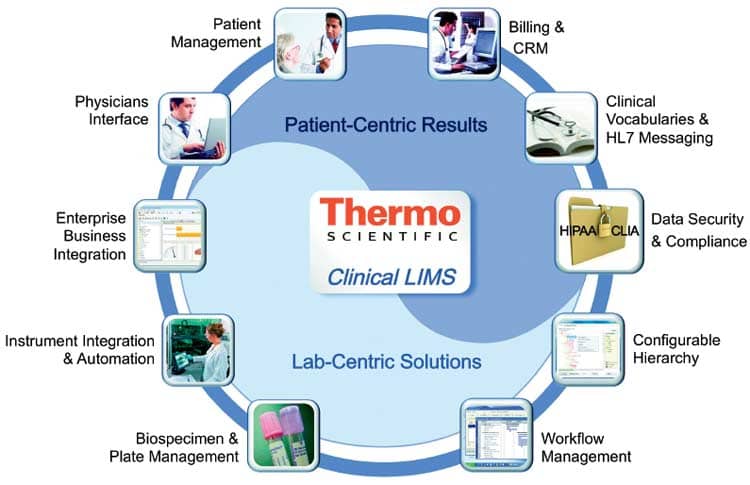
Thermo Scientific says its Clinical LIMS delivers both LIS and LIMS functionality and is suited to fill the gaps that have existed between the clinical research lab and the diagnostic environment. The Clinical LIMS is a comprehensive sample- and patient-management solution that enables physicians and health care staff to have pertinent data readily available to optimize patient care. This solution standardizes ordering and reporting practices and improves patient laboratory and health care documentation, allowing laboratories to manage health care information more efficiently and improve compliance with laws governing medical records management, the company says.
LAB-CENTRIC SOLUTIONS FOR PATIENT-CENTRIC RESULTS
So what is in store for the future? In 2006, Barack Obama, still a senator at the time, introduced the Genomics and Personalized Medicine Act. This bill, yet to be passed into law, has evolved a great deal over the past 5 years and defines its goal as: “to secure the promise of personalized medicine for all Americans by expanding and accelerating genomics research and initiatives to improve the accuracy of disease diagnosis, increase the safety of drugs, and identify novel treatments, and for other purposes.” If passed into law, Thermo Fisher says the industry could see major changes, such as the creation of an Office of Personalized Healthcare and a national biobank to manage human samples.
Regardless of what legislation is passed, there is little doubt that molecular diagnostic testing is a critical piece to achieving the goal of truly personalized care and that software is necessary to support that testing. “For personalized medicine to realize its goal of matching the right therapy to the right patient at the right time, the software used by those developing and delivering next-generation therapies must bring the right data to the right people at the right time,” Champagne says.
The key is to manage the laboratory information in a way that respects and maintains patient privacy, ensures data quality and traceability, and helps to deliver fast turnaround of test results to patients and physicians. Thermo Fisher says that by unifying the distinct laboratory and patient-management functionality needed by researchers and technicians involved in clinical testing and molecular diagnostics, Thermo Scientific Clinical LIMS delivers lab-centric solutions for patient-centric results.
OFFERING A BROAD-BASED PORTFOLIO
According to Clive Higgins, vice president of marketing for informatics at PerkinElmer, Waltham, Mass, the most significant change in regard to the scientific computing industry over the past couple of years in terms of mergers, acquisitions, and business changes is what PerkinElmer did with CambridgeSoft, ArtusLabs, and Labtronics by merging them into a complete broad-based portfolio of informatics offerings.
“The recent acquisitions extend PerkinElmer’s ELN and data integration software offerings into laboratories, such as regulated and nonregulated QA/QC, late-stage product or method development laboratories, and environmental and food testing labs,” Higgins says. “The combined offering allows PerkinElmer customers to rapidly access and share enterprise-wide data for faster, more informed scientific decisions.”
He notes that these changes happened because the ability to integrate scientific tools and informatics is becoming increasingly critical for PerkinElmer’s customers as they look to drive productivity and speed to market. “PerkinElmer is aware that our customers themselves have been going through a consolidation process of vendors, the numbers of systems used, and the architecture that they need to support, ultimately because of the costs associated,” Higgins says. “This is a continuation of what big businesses have been trying to do over the last 20 to 30 years by consolidating their supplier base. They would like to have fewer, but much deeper relationships with their vendors.
“PerkinElmer is moving into forming the infrastructure of these businesses, running the software and architecture to manage data internally and with external partners, as well,” Higgins adds. “We are driving toward increased productivity, which is exactly what our customers are asking for from vendors. The broader the capabilities, the more conversations the customers can have—from research to manufacturing—with a single provider.”
Higgins says there have been both positive and negative effects on his company’s business and on the range and quality of products and services on offer to customers.

PathCentral offers a Web-based LIS that cost-effectively integrates all of the critical components of a pathology practice’s day-to-day operations.
“Since March 2011, PerkinElmer has been integrating the three acquisitions and had 90% complementary services and instrumentation across the combined product portfolios,” he says. “We have manifested that overlap and merged our development strategy.” As an example, there was more than one ELN in the company’s combined portfolio following the acquisitions of Labtronics, ArtusLabs, and CambridgeSoft. PerkinElmer strategically managed to take the best functionalities from each, and combined them into a single platform called Ensemble, he says.
And Higgins says the firm’s smaller businesses, such as ArtusLabs and Labtronics, now have the expertise of a fully functional quality department, which is allowing quality to improve across the board. These businesses have the guidance of PerkinElmer, which has been providing quality instrumentation and services for almost 75 years. “This has enabled us to flesh out products that our customers truly need in order to increase productivity and speed,” Higgins says.
Over the next year, Higgins says he expects to see a continuation of growth in the area of biological data management, analysis handling, and a continued uptake of ELN use by biologists. “Previously, informatics was implemented mostly by chemists, but this is changing,” he says. “I think we’ll see the rollout of products using mobile technologies and the continued expansion of the adoption of iPads and similar platforms in labs—PerkinElmer included. The biggest impact to our customers is that businesses are now outsourcing their fundamental R&D activities to CROs.
“What that means is that instead of having everyone using the ELN infrastructure under one roof, the information is vastly distributed, worldwide,” Higgins says. “The infrastructure, security, and architecture that you have to build are quite different. So when a large pharmaceutical company does the initial discovery and sends a sample for assay development, which is done in China, that process needs to be totally seamless for the company’s informatics infrastructure. Yet it is not seamless in several ways. The sample has to be sent through DHL or FedEx, and it gets flown around the world, where assays are developed, with a whole range of logistical challenges like shipping and customs documentation. Lastly, the assay development data has to be returned to the original researchers in a single, seamless information environment. It’s what we call the externalization of R&D, and we’ve developed a unique architecture and product capabilities to deal with these challenges.”
Adds Dan Marshak, PerkinElmer chief scientific officer, “Our customers are people who are inundated by information, not only in the lab and within science but in their own homes as well,” he says. “We find that they have more questions than technology has allowed them to answer, and a lot more information to gather and decide on outcomes for different samples. They need help managing that information and the instrumentation and measurement technologies.
“In regard to consolidation, those groups need to integrate and, to large extents, understand what they have, who has it, etc,” Marshak, says. “Large organizations have plenty of knowledge, but it isn’t always accessible. The acquisitions were designed to create a complete portfolio of informatics and software solutions. We took a deep dive into making this a significant part of our business to enhance the company’s strategic focus on enterprise-wide knowledge-sharing and access.”

Clive Higgins, vice president of marketing, Informatics, PerkinElmer
WEB-BASED LIS SOLUTION FOR PATHOLOGISTS
The clinical diagnostics industry is no exception to the competitive pressures brought to bear by large companies. Large companies make it difficult for mom-and-pop operations to compete, and economy of scale is the watchword.
“For many years, community pathologists enjoyed steadily increasing revenues and attractive profit margins,” says Matt Watson, CEO, PathCentral Inc, Irvine, Calif. “But that started to change several years ago with the advent of the large regional and national anatomic pathology reference laboratories, and private community-based pathology practices have begun to feel the pinch.
Large companies have access to costly, sophisticated IT platforms and an extensive menu of leading-edge laboratory tests, giving them both a technological and financial edge, and enabling them to be highly effective in winning business away from the pathologists’ traditional client base—urologists, dermatologists, gastroenterologists, etc.
PathCentral aims to reverse the trend by giving community-based pathology practices a way to compete more effectively, Watson says.
Working as group administrator for a large, private pathology practice in the Los Angeles area in 2007, Watson began looking to gain access to the same powerful anatomic pathology laboratory information systems (APLIS) used so effectively by the large national companies, but at a cost local pathology groups could afford. He approached the owners of Mission Viejo, Calif-based eTeleNext, a company whose APLIS he had implemented years earlier at US LABS while serving as their vice president of operations. Watson says he learned that eTeleNext had established itself as the leading APLIS provider to the nation’s top reference laboratories. He and the eTeleNext team discussed the possibility of deploying the product using a software-as-a-services (SaaS) model; an approach sometimes referred to as cloud computing. By maintaining their patient information on cloud servers, pathology practices do not have to purchase and maintain expensive software and hardware. This savings would help make pathologists once again competitive in their local markets. And so much the better if the APLIS could be linked seamlessly to an esoteric diagnostics laboratory that would provide the pathologists access to the same menu of sophisticated send-out tests their competitors were offering.
After nearly 2 years of planning and negotiations, Watson and eTeleNext teamed in 2009 to form PathCentral. Its mission is to help community-based pathology practices increase revenue, reduce operating costs, and become more competitive in their local markets. PathCentral developed a Web-based LIS that cost-effectively integrates all of the critical components of a pathology practice’s day-to-day operations, including accessioning, bar code-based specimen tracking, grossing, histology, transcription, test send-outs, second opinions/expert consultations, reporting, billing, and financial analysis. Because the system is Web-based, there is no up-front license fee and no hardware or software to purchase and maintain. Customers pay a monthly subscription fee and get unlimited access to a state-of-the-art enterprise pathology system, as well as 24/7/365 service and support, system maintenance, and software version upgrades. The system features the latest in information technology, including an open-Web service layer, which enables it to easily connect with hospitals, physicians’ offices, billing companies, and a wide variety of laboratory instrumentation. “We’re ahead of the curve with respect to connectivity, and that’s becoming a critically important issue for pathologists,” Watson says.

Matt Watson, CEO, PathCentral Inc
One entity the pathology practice would like to be electronically connected to is its reference laboratory. “The method for ordering send-out tests is still very archaic,” Watson says. If a pathologist needs to order a test on a breast cancer case, for example, even though all of the patient’s information may exist in her laboratory’s LIS, she still must manually enter all of that information into a hard-copy test requisition, select the test to be ordered, and send the paper requisition along with the patient specimen to the reference laboratory. When the package arrives at the reference laboratory, a clerk must re-enter the patient information into yet another computer system before the test can be performed. Finally, when the test results are available, they must be manually entered into the original ordering pathologist’s LIS before a final report can be generated. “This process of multiple data entry steps is slow and inefficient, and can create patient safety and billing issues,” Watson says. PathCentral has created a system that gives clients a simple means to electronically accession a patient, order a send-out test, and receive results back, all with a single data entry step, he says. The system is efficient because all parties—the clinician, the pathologist, and the reference lab—are using the same software platform. The system also gives community pathologists access to a comprehensive menu of send-out tests through the company’s esoteric diagnostics laboratory, he says.
PathCentral is focused on providing leading-edge genomic tests to its customers and has attracted a team of laboratory professionals with core competency in FISH, PCR, array CGH, and DNA sequencing technologies. Watson is quick to point out that PathCentral does not employ any pathologists. “We don’t want to appear to be competing with our own customers,” he says. “We offer only technical component services and allow our clients to do the analysis and interpretation (the “professional component”) and bill the payor for those services. This is another way we help our pathology customers increase their practices’ revenue.
In addition to the APLIS and laboratory testing services, PathCentral also assists its clients in deploying a courier network, hiring and training sales representatives, and developing high-impact marketing materials. Through its professional pathology network, scheduled to launch in the first quarter of 2012, PathCentral will provide its customers with seamless, online access to world-renowned subspecialty experts for those cases that require a second opinion or professional consultation, Watson says. “No longer will a pathologist have to package glass slides, ship them to a consultant hundreds or thousands of miles away, and wait several weeks for a hard-copy report to come back in the mail,” he says. “Using our online network, PathCentral’s clients will be able to scan a slide and make a high-magnification, high-resolution image of that slide available to the consultant with a simple click of a mouse. Once the consultant has completed her assessment of the case, she’ll be able to instantly send an electronic report back to the ordering pathologist. This saves time and money, and improves patient care,” Watson says.
Gary Tufel is a contributing writer for CLP.