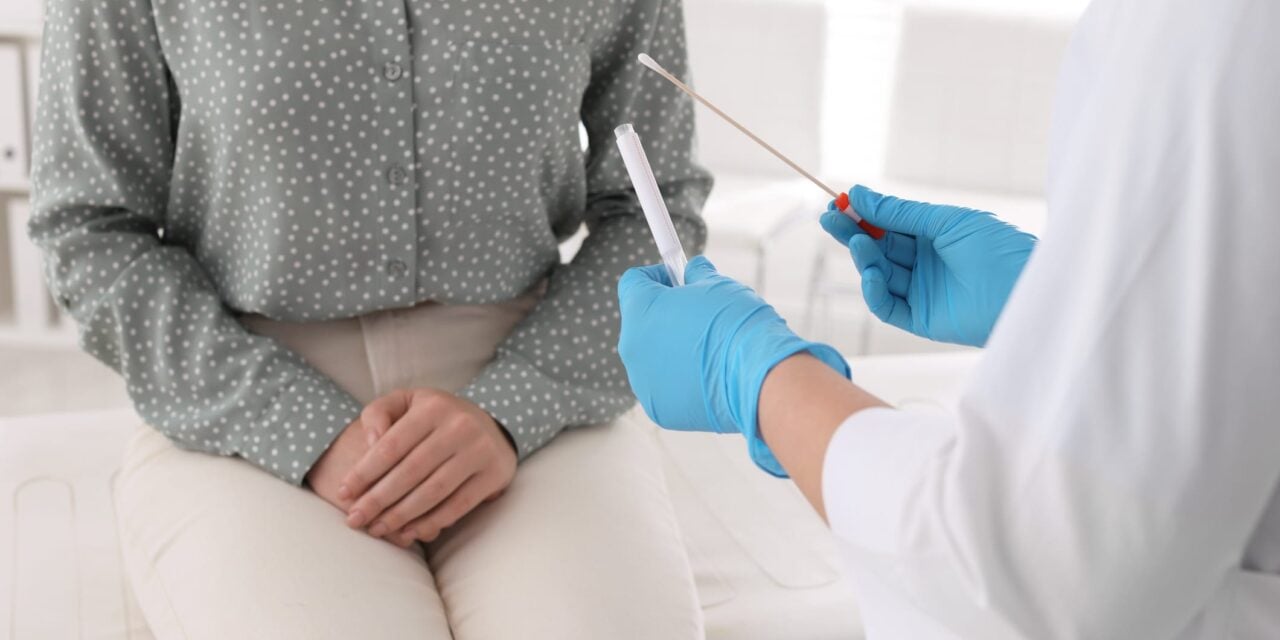
The new guidelines allow self-collected vaginal samples for HPV testing and outline criteria for safely discontinuing cervical cancer screening.
The American Cancer Society (ACS) has released updated guidelines for cervical cancer screening, introducing two key changes: self-collection of vaginal samples for human papillomavirus (HPV) testing as an option for cervical cancer screening and new guidance on when individuals can safely exit screening.
The updated guideline for women at average risk and individuals with a cervix at average risk reflects advances in disease detection and accessibility. The recommendations are published in CA: A Cancer Journal for Clinicians.
“These updated recommendations will help to improve compliance with screening and reduce the risk of cervical cancer,” says Robert Smith, PhD, senior vice president of early cancer detection science at the American Cancer Society and senior author of the report, in a release. “They are made possible as we continue to evolve our approach to screening for cervical cancer, primarily through research advancements, and the development of self-collection tools to broaden access to screening.”
Key Changes to Screening Recommendations
For primary HPV testing, clinician-collected cervical specimens remain preferred, but self-collected vaginal specimens are now acceptable for cervical cancer screening. When self-collected vaginal specimens are HPV negative in the screening setting, repeat testing in three years is recommended.
The ACS continues to recommend that average-risk women and individuals with a cervix at average risk initiate cervical cancer screening at age 25 and undergo primary HPV testing every five years through age 65. If primary HPV testing is not available, individuals ages 25-65 should be screened by co-testing with an HPV test combined with a cytology (Pap) test every five years, or cytology testing alone every three years.
For discontinuing screening, ACS now recommends that average-risk individuals have negative primary HPV tests or negative co-testing using HPV tests and cytology testing at ages 60 and 65. If primary HPV tests or co-testing are not available, three consecutive negative cytology tests at the recommended screening interval, with the last test at age 65, are acceptable.
Clinical Impact and Access Considerations
The changes follow US Food and Drug Administration approval of HPV self-collection testing as a safe and effective screening option. Cervical cancer screening programs have decreased cancer incidence by more than half since the mid-1970s, but 13,360 cases are expected to be diagnosed in the US this year, with an estimated 4,320 deaths from the disease.
“Geographic disparities continue to exist in cervical cancer incidence and mortality, with individuals living in rural areas more likely to be diagnosed with later-stage cervical cancer. Over 46 million, or 14%, of the US population live in rural areas that often require the need to travel long distances to access health care,” says Lisa Lacasse, president of the ACS Cancer Action Network, in a release. “Self-collection options are a critical resource for these individuals and other underserved populations.”
The updated report includes a patient page supporting the new guideline, providing evidence-based medical content to help patients understand screening options and treatment approaches.
The guideline development process involved ongoing monitoring of medical and scientific literature for new evidence by ACS scientists and volunteers. Research has shown that long-lasting infection with certain types of HPV causes nearly all cervical cancers.
ID 239964962 © Chernetskaya | Dreamstime.com