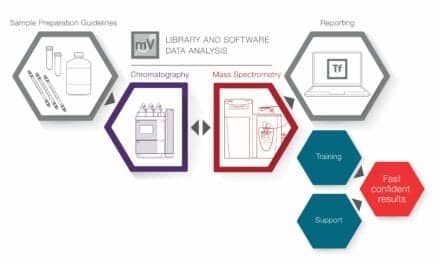
Labcorp to Implement Roche’s Automated Mass Spectrometry Platform
Labcorp will deploy Roche’s cobas Mass Spec platform in its US labs, marking the first commercial rollout of this fully automated mass spectrometry system to streamline complex diagnostic testing workflows.