Tempus Launches AI-Powered Digital Pathology Suite for Biomarker Prediction
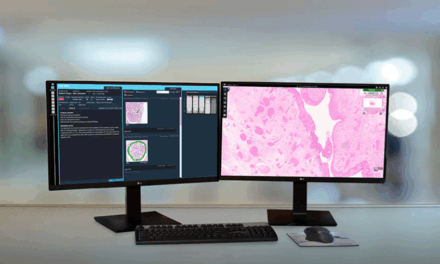
Tempus has introduced Paige Predict, an AI-powered pathology suite that analyzes H&E slides to predict 123 biomarkers across 16 cancer types when tissue samples are insufficient.