Home AI Blood Testing Device Gains FDA Clearance for Point of Care
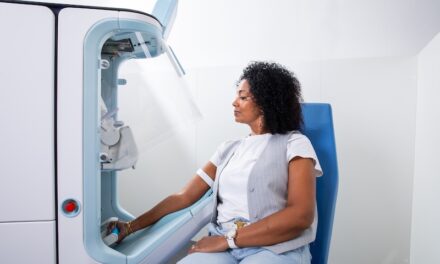
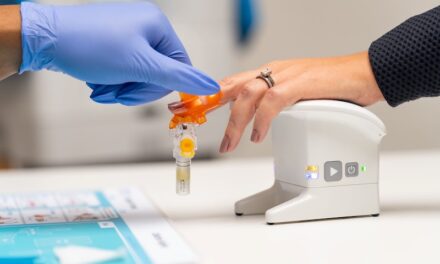
The FDA has cleared Athelas Home’s AI-powered hematology analyzer for point-of-care use, enabling rapid white blood cell testing from fingerstick samples in ambulatory clinics and pharmacies.